Diffusion
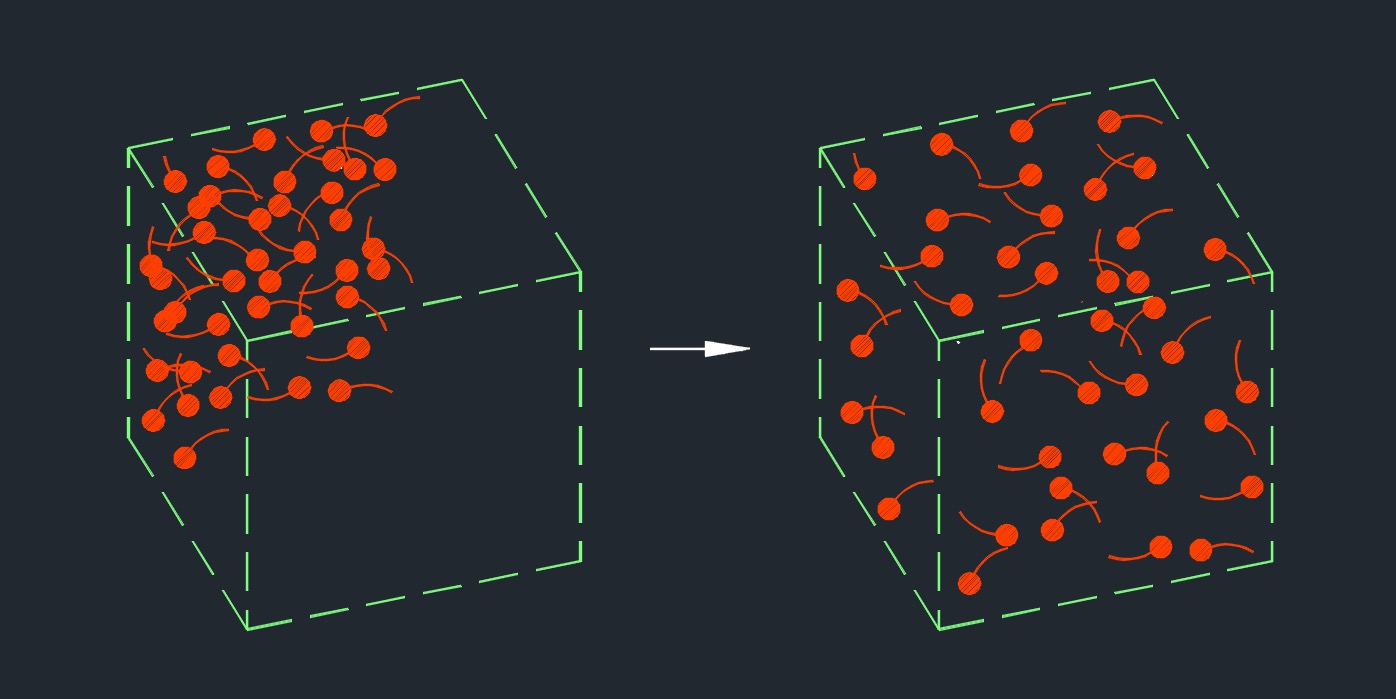 Diffusion refers to the spontaneous movement of particles or molecules from an area of higher concentration to an area of lower concentration. Diffusion occurs due to the random thermal motion of particles. In a system where there is a concentration gradient, particles move in a manner that tends to equalize the concentration throughout the system. This movement is driven by the principle of maximizing entropy, as particles disperse to increase their disorder and achieve a more uniform distribution.
Diffusion refers to the spontaneous movement of particles or molecules from an area of higher concentration to an area of lower concentration. Diffusion occurs due to the random thermal motion of particles. In a system where there is a concentration gradient, particles move in a manner that tends to equalize the concentration throughout the system. This movement is driven by the principle of maximizing entropy, as particles disperse to increase their disorder and achieve a more uniform distribution.
The rate of diffusion depends on several factors, including the concentration gradient, temperature, surface area, and the properties of the diffusing species and the medium through which diffusion occurs. In general, larger concentration gradients, higher temperatures, and larger surface areas facilitate faster diffusion.
Diffusion is an essential mechanism in many natural and industrial processes. It influences the transport of substances in biological systems, the spread of pollutants in the environment, the mixing of fluids in chemical reactions, and the behavior of materials in solid-state processes. The study of diffusion is crucial for understanding these processes and developing strategies to control and manipulate diffusion rates in various applications. It is a fundamental process that plays a crucial role in various scientific disciplines, including physics, chemistry, biology, and materials science.
