Freezing
 Freezing is the process of changing the state of a substance from a liquid to a solid as a result of removing heat. When a liquid is cooled, the molecules or atoms within it lose kinetic energy and begin to slow down. If the temperature of the liquid is lowered below its freezing point, the intermolecular forces between the particles become strong enough to hold them in a rigid structure, resulting in the formation of a solid. The freezing point of a substance is a physical property that is specific to each substance and is dependent on factors such as pressure and purity. The freezing point can be used as a tool to identify and purify substances.
Freezing is the process of changing the state of a substance from a liquid to a solid as a result of removing heat. When a liquid is cooled, the molecules or atoms within it lose kinetic energy and begin to slow down. If the temperature of the liquid is lowered below its freezing point, the intermolecular forces between the particles become strong enough to hold them in a rigid structure, resulting in the formation of a solid. The freezing point of a substance is a physical property that is specific to each substance and is dependent on factors such as pressure and purity. The freezing point can be used as a tool to identify and purify substances.
Freezing is an important process in many industrial and scientific applications. It is used in food preservation to prevent spoilage by slowing or stopping the growth of bacteria, and in cryopreservation to preserve biological samples such as cells and tissues. It is also an important process in the Earth's geology, where the freezing of water in the ground can lead to the formation of permafrost and the expansion of ice can cause physical weathering of rocks.
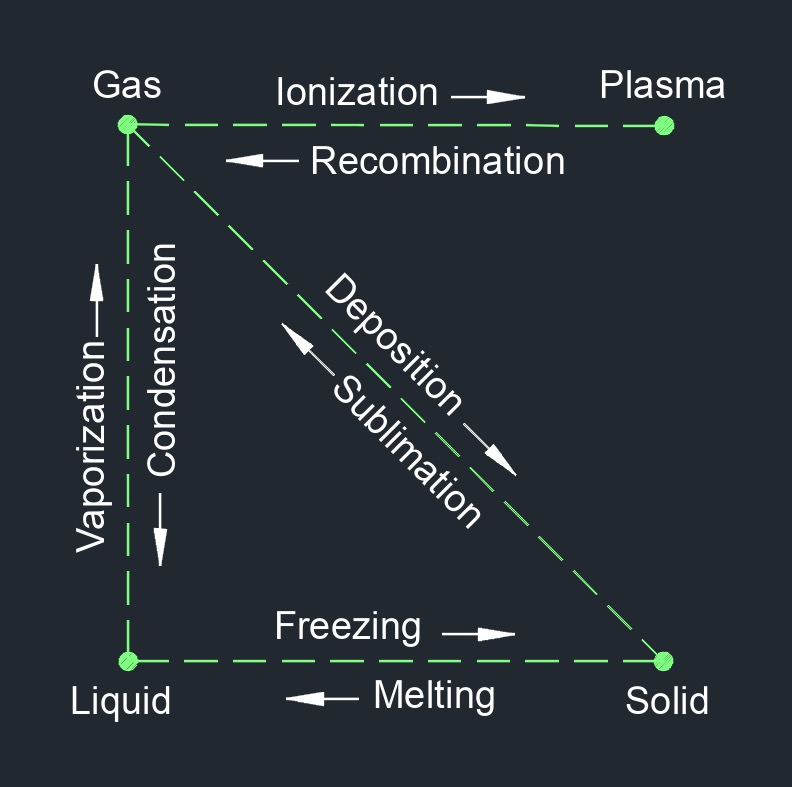
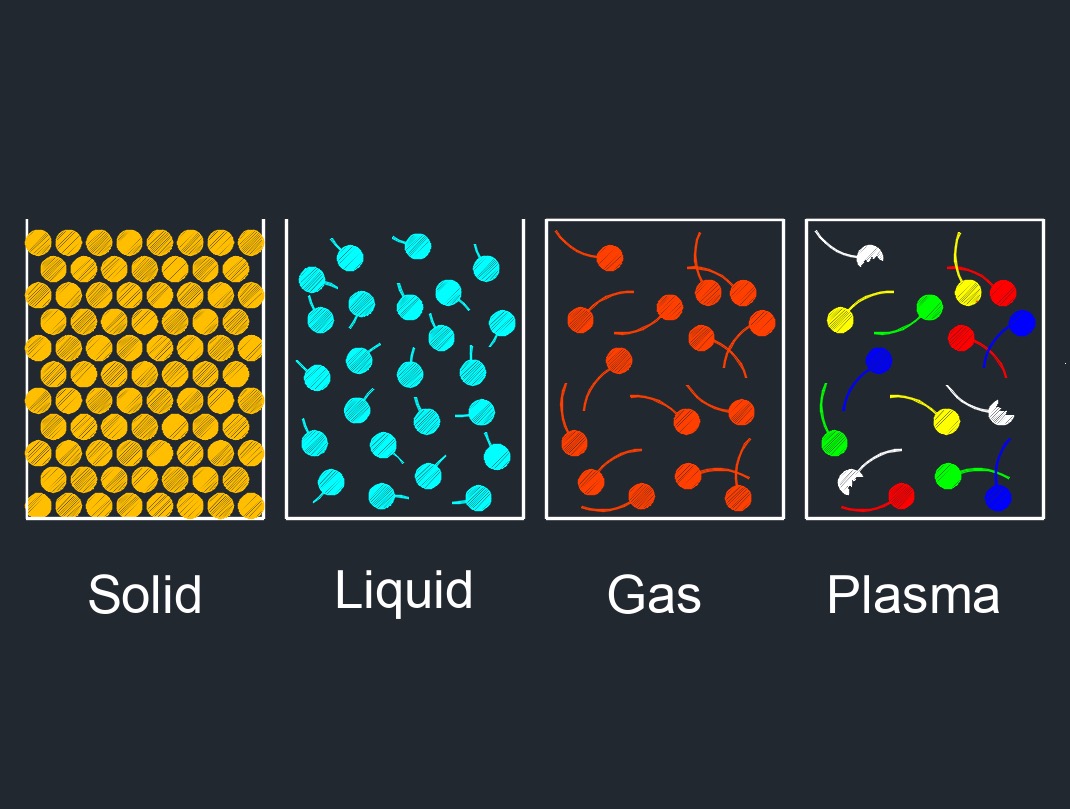
There are four phases of matter: gas, liquid, plasma, and solid.

Tags: Temperature Matter