First Law of Thermodynamics
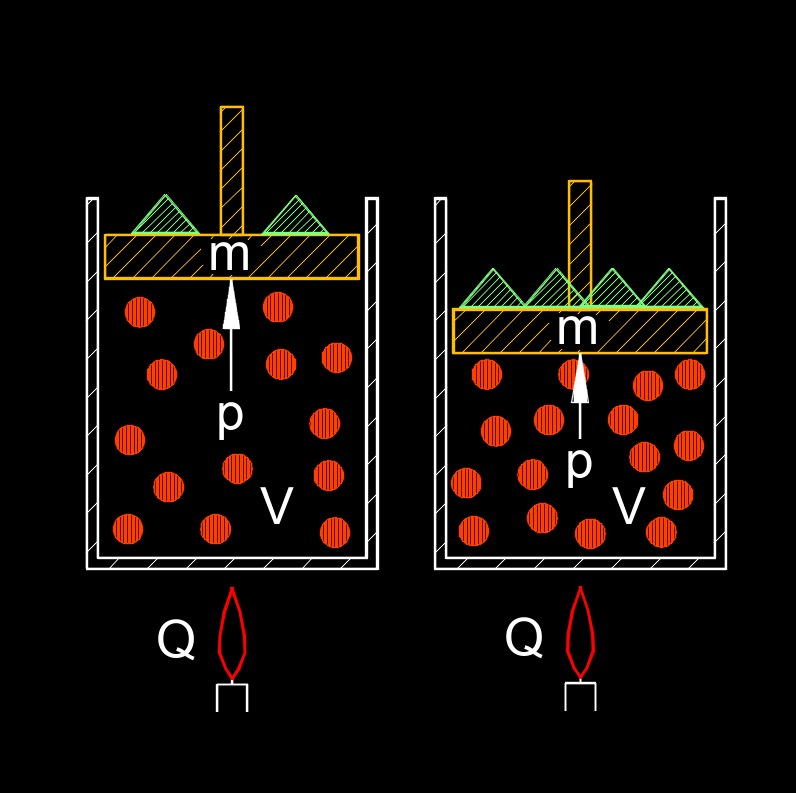 The first law of thermodynamics (also called conservation of energy) expresses the principal of the conservation of energy. This means that the total amount of energy in the universe is constant and that it can neither be created or destroyed. This law states that for every gain in some type of energy will result in the loss in some other form.
The first law of thermodynamics (also called conservation of energy) expresses the principal of the conservation of energy. This means that the total amount of energy in the universe is constant and that it can neither be created or destroyed. This law states that for every gain in some type of energy will result in the loss in some other form.
In general, the conservation of energy can be stated as follows - Kinetic Energy + Potential Energy = Constant
- \( p \; V = n \; R \; T \) (Ideal Gas Law)
- \( W = p \; \Delta V \) (p is constant)
- \( W = \int \; p \; d V \) (p not constant)
The following two formulas are the same and the law can be expressed as either. The first formula is normally the one used.
First Law of Thermodynamics formulaThe change in the internal energy of the gas = heat added to the gas - work done "by" the gas. |
||
|
\( \Delta U = Q - W \) (First Law of Thermodynamics) \( Q = \Delta U + W \) \( W = Q - \Delta U \) |
||
| Symbol | English | Metric |
| \(\triangle U\) = internal energy | \(lbf-ft\) | \(J\) |
| \(Q\) = heat added to the system | \(F\) | \(C\) |
| \(W\) = work done by the system | \(lbf-ft\) | \(J\) |
First Law of Thermodynamics formulaThe change in the internal energy of the gas = heat added to the gas + work done "on" the gas. |
||
|
\( \Delta U = Q + W \) (First Law of Thermodynamics) \( Q = \Delta U - W \) \( W = \Delta U - Q \) |
||
| Symbol | English | Metric |
| \(\triangle U\) = internal energy | \(lbf-ft\) | \(J\) |
| \(Q\) = heat added to the system | \(F\) | \(C\) |
| \(W\) = work done by the system | \(lbf-ft\) | \(J\) |


