Vaporization
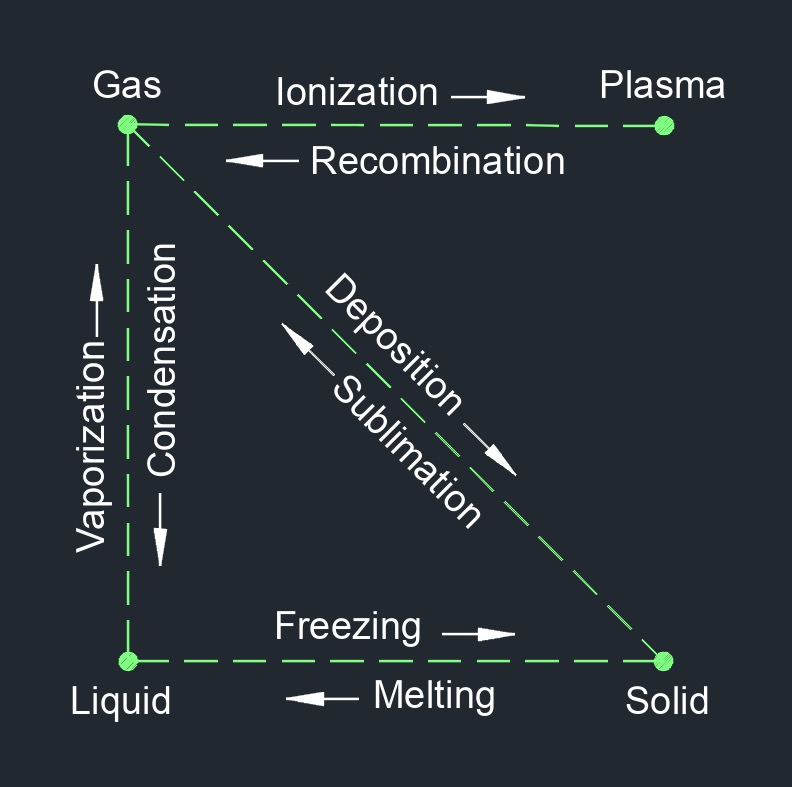
 Vaporization is a physical change of a substance from a liquid phase to a gas phase. Most substances undergo a change of state from solid to liguid to a gaseous state as the temperature rises. When a liquid is changed to a gas, the process is either called vaporization or boiling. The difference between boiling and evaporation is that boiling is below the surface of the liquid, and evaporation is at the surface of the liquid.
Vaporization is a physical change of a substance from a liquid phase to a gas phase. Most substances undergo a change of state from solid to liguid to a gaseous state as the temperature rises. When a liquid is changed to a gas, the process is either called vaporization or boiling. The difference between boiling and evaporation is that boiling is below the surface of the liquid, and evaporation is at the surface of the liquid.
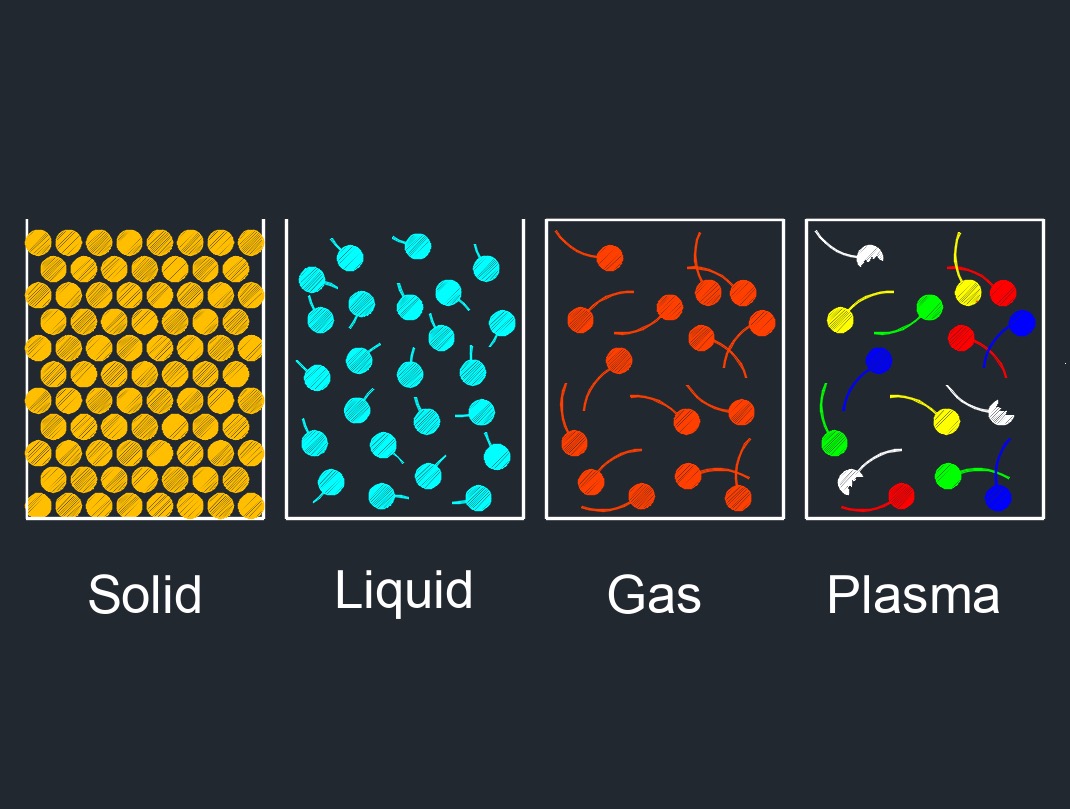
There are four phases of matter: gas, liquid, plasma, and solid.
Steam Temperatures
Dry Steam (Superheated Steam) \(\;\;>100 \; ^\circ C\) \((>212 \; ^\circ F)\)
Saturated Steam \(\;\;100 \; ^\circ C\) \((212 \; ^\circ F)\)
Wet Steam (Unsaturated Steam) \(\;\;100 \; ^\circ C\) \((212 \; ^\circ F)\)
Water \(\;\;<100 \; ^\circ C\) \((<212 \; ^\circ F)\) Vaporization Starts
Ice \(\;\;0 \; ^\circ C\) \((32 \; ^\circ F)\)

