Gay-Lussac's Law
Gay-Lussac's Law Formula |
||
|
\( \dfrac{ p_1 }{ T_1} \;=\; \dfrac{ p_2}{T_2 }\) (Gay-Lussac's Law) \( p_1 \;=\; \dfrac{ T_1 \cdot p_2}{T_2 }\) \( T_1 \;=\; \dfrac{ p_1 \;\cdot T_2}{p_2 }\) \( p_2 \;=\; \dfrac{ p_1 \cdot T_2}{T_1 }\) \( T_2 \;=\; \dfrac{ T_1 \cdot p_2}{p_1 }\) |
||
| Symbol | English | Metric |
| \( p_1 \) = Pressure | \(lbf\;/\;in^2\) | \(Pa\) |
| \( T_1 \) = Absolute Temperature | \(^\circ F \) | \(^\circ K \) |
| \( p_2 \) = Gas Pressure | \(lbf\;/\;in^2\) | \(Pa\) |
| \( T_2 \) = Absolute Temperature | \(^\circ F \) | \(^\circ K \) |
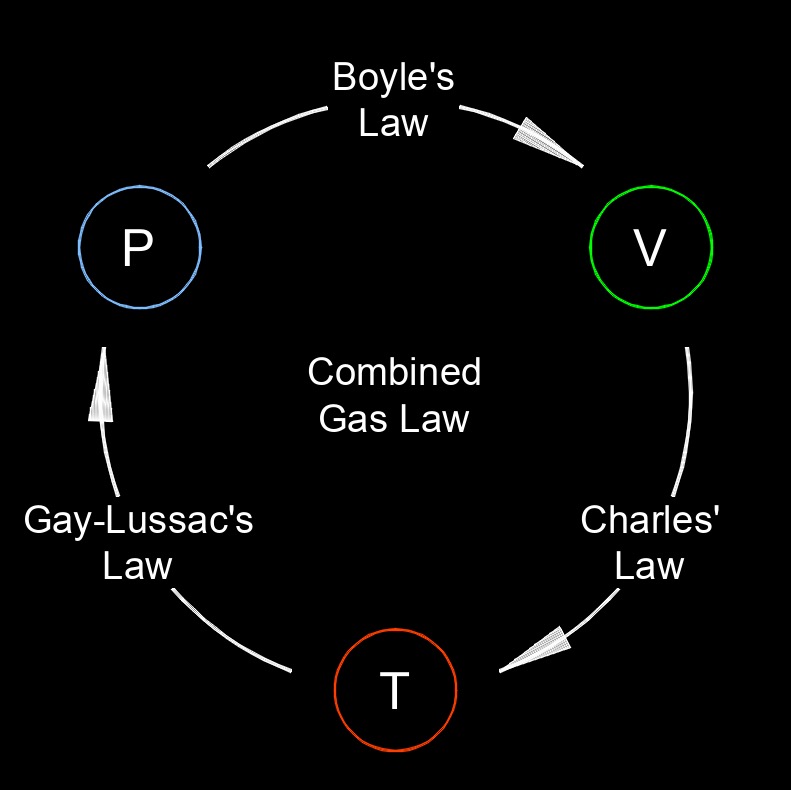 Gay-Lussac's law, also called pressure-temperature law or pressure law, states that the pressure of a gas is directly proportional to its temperature, when the volume and the amount of gas remain constant. This law describes the relationship between pressure and temperature for a given amount of gas.
Gay-Lussac's law, also called pressure-temperature law or pressure law, states that the pressure of a gas is directly proportional to its temperature, when the volume and the amount of gas remain constant. This law describes the relationship between pressure and temperature for a given amount of gas.
According to Gay-Lussac's law, if the temperature of a gas increases while the volume and the amount of gas remain constant, the pressure of the gas also increases. Similarly, if the temperature decreases, the pressure decreases proportionally. This direct relationship between pressure and temperature holds as long as other variables, such as volume and the amount of gas, remain constant.
Gay-Lussac's law provides an insights into the behavior of gases and finds applications in various fields, such as gas thermodynamics, industrial processes, and engineering. Additionally, Gay-Lussac's law is a key component of the combined gas law, which combines Gay-Lussac's law with Boyle's law and Charles' law to describe the relationship between pressure, volume, and temperature in a gas.

