Thermodynamics
Thermodynamics is the branch of physics that deals deals with large scale reactions of a system that can be observed and measures and the relationship between properties such as energy, heat, pressure, temperature, work, etc. on a system. It is a fundamental part of physics and is used to describe and understand the behavior of many different physical systems, ranging from tiny particles to large-scale processes like weather patterns and the behavior of stars.
Thermodynamic Index
The laws of thermodynamics describe the behavior of energy in different systems. The first law states that energy cannot be created or destroyed, only converted from one form to another. The second law states that the total entropy (a measure of the degree of disorder in a system) in a closed system will always increase over time, and that energy will always tend to flow from hotter to cooler objects. The third law states that it is impossible to reach absolute zero (the lowest possible temperature).
Thermodynamics has many practical applications, including the design of engines, refrigeration systems, and other devices that convert energy from one form to another. It is also important in chemistry, where it is used to study the behavior of chemical reactions and the properties of materials at different temperatures and pressures.
Science Branches |
| Science |
| Natural Science |
| Physical Science |
| Physics |
| Classical Physics |
| Mechanical Physics |
| Thermodynamics |
Article Links |
Association Links |
Mathematics Links |
Tag Links
|
Nomenclature & Symbols |
System Types
- Boundary - The real or imaginary surface that separates the system ftom the surroundings and can be fixed or movable.
- Closed System - Exchanges only energy with its surroundings. No mass can cross the system.
- Isolated System - Keeps the energy and matter within the system and everything else out. No transfer in or out.
- Open System - Freely exchanges energy and matter with its surroundings. Both mass and energy can cross the boundary.
Properties of a System
The identifiable and observable characteristics of a system by which it can be specified.
- Extensive Properties - Those properties which depend on the size of the system. These properties are directly related to the mass.
- Enthalpy
- Entropy
- Gibbs Free Energy
- Heat Capacity
- Internal Energy
- Mass
- Volume
- etc.
- Intensive Properties - Those properties of the system which do not depend on the size of the system. These properties are not related to the mass.
- Color
- Density
- Pressure
- Temperature
- Thermal Expansion
- Velocity
- Viscosity
- etc.
Properties of a System
The identifiable and observable characteristics of a system by which it can be specified.
- Extensive Properties - Those properties which depend on the size of the system. These properties are directly related to the mass.
- Enthalpy
- Entropy
- Gibbs Free Energy
- Heat Capacity
- Internal Energy
- Mass
- Volume
- etc.
- Intensive Properties - Those properties of the system which do not depend on the size of the system. These properties are not related to the mass.
- Color
- Density
- Pressure
- Temperature
- Thermal Expansion
- Velocity
- Viscosity
- etc.
Thermodynamic Processes
Any process that involves heat energy moving within a system or between systems.
- Adiabatic Process - No heat is transferred to or from the system.
- Cyclic Process - The system at the end is the same as the state at the beginning. The system does not change over a complete cycle.
- Irreversible Process - Cannot return both the system and the surroundings to their original conditions.
- Isenthalpic Process - A process of constant enthalpy.
- Isochoric Process - The system’s volume does not change.
- Isobaric Process - The system’s pressure does not change.
- Isothermal Process - The system’s temperature remains constant.
- Non-quasi-static Process - Most of the processes happening around us can be termed non-quasi-static process.
- Quasi-static Process - Slow enough for the system to maintain internal thermodynamic equilibrium.
- Reversible Process - Can be made to retrace its path by differential changes in the environment. It leaves no change in either the system or surroundings.
Thermodynamics Glossary
A
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Absolute Entropy - Is entropy calculated relative to the absolute reference point determined by the Third Law of Thermodynamics.
- Absolute Humidity - The mass of water vapor divided by the mass of dry air in a certain volume of air at a specific temperature.
- Absolute Pressure - A pressure at absolute zero can only exist in a total vacuum and any pressure above this is called absolute pressure.
- Absolute Temperature - Is measured from the starting point of 0, where zero is the coldest theoretically attainable temperature in nature.
- Absolute Zero - The temperature at which all motion within molecules completely stops. Below absolute zero temperature does not exist.
- Adiabatic Compression - The external work done is equal to the increased internal energy of the air in the system. Here the heat is neither subtracted or added from the surrounding air to the system air. As there is an increase in the temperature of the system the pressure of the system tends to be more than the volume.
- Adiabatic Expansion - The ideal behaviour of a system where the temperature keeps on increasing and pressure remains constant. It refers generally to a closed system.
- Adiabatic Heat Drop - The heat energy released and theoretically capable of transformation into mechanical work during the adiabatic expansion of unit weight of steam or other vapour of gas.
- Adiabatic Process - An adiabatic process describes a process that remains aloof from its surroundings. It is a process in which no heat transfer occurs between a system and its surroundings. Here, the temperature of the system can vary in order to avoid any heat transfer.
- Air - A gas consisting principally of a mixture ot 78% nitrogen, 22% oxygen, 1% argon, and the other 4% minor gasses like approximately 0.4% carbon dioxide, 0.08% neon, 0.005% helium, 0.0001% krypton, 0.00005% hydrogen, and trace amounts of xenon gas. The composition can vary according to local conditions.
- Amagat's Law - States that the volume of a mixture is equal to the sum of the partial volume of its components.
- Ambient Temperature - The current surrounding environment air temperature. This temperature has nothing to do with high or low forcasts.
- Antoine Equation - The relationship between vapor pressure and the temperature of pure substances.
- Area Thermal Expansion - Happens when any change in temperature expands the area.
- Area Thermal Expansion Coefficient - The ratio of the change in size of a material to its change in temperature.
- Arrhenius Equation - The temperature dependance of the reaction rate constant which is the rate of chemical reaction.
- Atmospheric air - The air in the atmosphere, which normally contains some water vapor.
B
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Boltzmann Constant - Relates the average kinetic energy of molecules of a gas to its temperature.
C
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Celsius - A unit of temperature most commonly used through out the world.
- Charles' Law - , one of the gas laws, states that at a constant pressure the volume of a given mass of gas is directly porportional, increases or decreases, to the absolute temperature.
- Chemical Energy - Is when two substances are combined or mixed together (atoms and molecules interacting) to produce a chemical reaction.
- Clausius-Clapeyron Equation - The vapor pressure of given liquids or solids. This allows us to estimate the pressure temperature, if the vapor pressure is known at some temperature and if the enthalpy of vaporization is known.
- Clean Steam - Steam that is generated from pure water, free from contaminants and impurities.
- Closed System - Exchanges only energy with its surroundings, not matter.
- Coefficient of Aerial Thermal Expansion - See Area Thermal Expansion Coefficient
- Coefficient of Compressibility - See Compressibility
- Coefficient of Linear Thermal Expansion - See Linear Thermal Expansion coefficient
- Coefficient of Thermal Expansion - See Thermal Expansion Coefficient
- Coefficient of Volumetric Thermal Expansion - See Volumetric Thermal Expansion Coefficient
- Cold - Is when the temperature is less than normal or the lack of heat.
- Combustion - A reaction called rapid oxidation or burning produced with the right combination of a fuel, oxygen, and heat.
- Combustion Efficiency - The amoount of heat released during combustion divided by the heating value of the fuel.
- Complete Combustion - A combustion process in which all the carbon in the fuel burns to \(CO_2\), all the hydrogen burns to \(H_2O\), and all the sulfure (if any) burns to \(SO_2\).
- Compression Ratio - The ratio of the maximum volume to the minimum volume in a cylinder.
- Compressibility - Measures the change in volume under external forces for any liquid.
- Compressor - A device that forces air or gas into a smaller area increasing the volume and creating a usable force of energy.
- Condensation - A physical change of a substance from a gas phase to a liquid phase. The molecules are fast moving and far apare.
- Condenser - An outdoor part of the AC or heat pump that releases or collects heat.
- Conduction Heat Transfer - See Heat Transfer by Conduction
- Conductor - A material through which heat passes.
- Conservation of Energy - See First Law of Thermodynamics
- Convective Heat Transfer - See Heat Transfer by Convection
- Convective Heat Transfer Coefficient - A porportional constant between heat flux and force from the flow of heat, this also depends on the type of fluid and fluid velocity.
- Critical Point - The point at which the saturated liquid and saturated vapor states are identical.
- Critical Pressure - The ctitical pressure of a vapor is the pressure required to liquefy it at the critical temperature and is the highest pressure on the temperature-pressure graph for standard vapor.
- Critical Properties - The properties of a fluid at a location where Mach number is unity.
- Critical Ratio - The ratio of the stagnation to static properties when the Mach number is unity.
- Critical State of a Substance - The state at which liquid and vapor coexist in equilibrium. At critical state, latent heat of vaporization becomes zero.
- Critical Temperature - The temperature of the vapor above which no pressure, however high, will produce liquefaction.
- Critical Velocity - The velocity above which fluid flow is turbulent.
- Critical Volume - The volume of substance at the critical point.
D
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Dead State - When a system is in thermodynamic equilibrium with the envitonment.
- Deficiency of Air - When the amount of air is less than the stoichiometer amount.
- Density - The ratio of the amount of matter in an object compared to its volume.
- Density of an Ideal Gas - Greatly affected by pressure.
- Deposition - A physical change of a substance from a gas phase to a solid phase and does not pass through the transitional liquid phase. This is exactly the opposite of sublimation.
- Discharge Coefficient - The ratio of actual discharge to the theoretical discharge.
- Dry Air - Air that contains no water vapor.
- Dry Bulb Temperature - The actual temperature of air.
- Dynamic Temperature - The kinetic energy per unit mass divided by the constant pressure specific heat and corresponds to the temperature rise during the stagnation process.
E
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Efficiency - Is expressed in percentage and always less than 100%.
- Elastic Modulus - See Young's Modulus
-
Emissivity - The surface depends on the material effectiveness in emitting energy as thermal radiation and varies between 0.0 and 1.0.
- Energy - Is never created or destroyed First Law of Thermodynamics, but it can be transferred from one object to another.
- Enthalpy - Measures the sum of internal energy changes in heat under constant pressure of the system.
- Enthalpy of Combustion - The enthropy of reaction during a steady-flow combustion process when 1 kmol (or 1 kg) of fuel is burned completely at a specific preasure and temperature and represents the amount of heat released.
- Enthalpy Departure - The difference between the enthalpy of a real gas and the enthalpy of the gas at an ideal state and it represents the variation of the enthalpy of a gas with pressure at a fixed temperatiure.
- Enthalpy Departure Factor - The difference between the entropy of a real gas at a given pressure and temperature and the entropy of the gas at an ideal gas state at the same pressure and temperature.
- Enthalpy of Formation - The enthalpy of a substance at a specific state due to its chemical composition.
- Enthalpy of Reaction - The difference between the enthalpy of the products at a specific state and the enthalpy of the reactants at the same state for a complete reaction.
- Entropy - Measures the unavailable energy in a heat system.
- Entropy Change of a Closed System - The entropy transfer accompanting heat transfer and the entropy generation within the system boundaries.
- Equilibrium - In a equilibrium state there are no unbalanced potentials, or driving forces within the system.
- Evaporation - The condition that occurs when heat is absorbed by the surface of a liquid and it changes to vapor.
- Evaporative Condenser - Combines the principles of force circulation convection currents with the ability of a vaporizing liquid to absorb heat.
F
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Fahrenheit - A unit of temperature used in the United States and a few other countries.
- Film Coefficient - See heat transfer coefficient
- Film Effectiveness - See heat transfer coefficient
- Fire Point - Lowest temperature at which a combusible fluid will burst into flame in the presence of an foreign ignition source.
- First Law of Thermodynamics - The total amount of energy in the universe is constant and that it can neither be created or destroyed.
- Flash point - The temperature at which combustion is initiated.
- Flash Steam - When hot condensate is released from a high pressure to a lower pressure steam.
- Fouling - Accumulation of unwanted deposits on heat transfer surfaces. This creates an additional barrier for heat movement, reduces heat transfer efficiency.
- Fourier's Law - When there exists a temperature gradient within an object, heat energy will flow from the high temperature region to the low temperature region.
- Fourier's Law of Thermal Conduction - See Fourier's Law
- Freezing - A physical change of a substance from a liquid phase to a solid phase.
G
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Gas - Able to be compressed to fit a confined space and expanded when released.
- Gas Compressibility Factor - A factor independent of the quantity of gas and determined by the character of the gas, the temperature, and pressure.
- Gas Laws - One of the four phases of matter (gas, liquid, plasma, and solid). These laws deal with the behavior of gas with respect to pressure, temperature, and volume.
- Gay-Lussac's Law - One of the Gas Laws, states the proportional, that when temperature increases, pressure increases, when pressure decreases, temperature decreases.
- Gauge Pressure - the difference between the absolute pressure and the local atmospheric pressure.
- Geothermal Heat Pump - Uses the ground as the heat source.
H
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Heat - A form of energy that causes physical change in what is being heated. The lack of heat is cold.
- Heat Capacity - The ratio of heat transferred to raise the temperature of an object.
- Heat Capacity at Constant Pressure - At constant pressure, some of the heat goes to doing work. The value of heat capacity depends on whether the heat is added at constant volume, constant pressure, etc.
- Heat Capacity at Constant Volume - At a constant volume, all the heat added goes into raising the temperature. The value of heat capacity depends on whether the heat is added at constant volume, constant pressure, etc.
- Heat Conduction - See Heat Transfer by Conduction
- Heat Convection - See Heat Transfer by convection
- Heat-driven System - Refrigerator systems whose energy input is based on heat transfer from an external source.
- Heat Energy - See Thermal Energy
- Heat Exchanger - A device used to transer heat from one medium to another at different temperatures. The heat transfer can be air or a liquid such as water or oil.
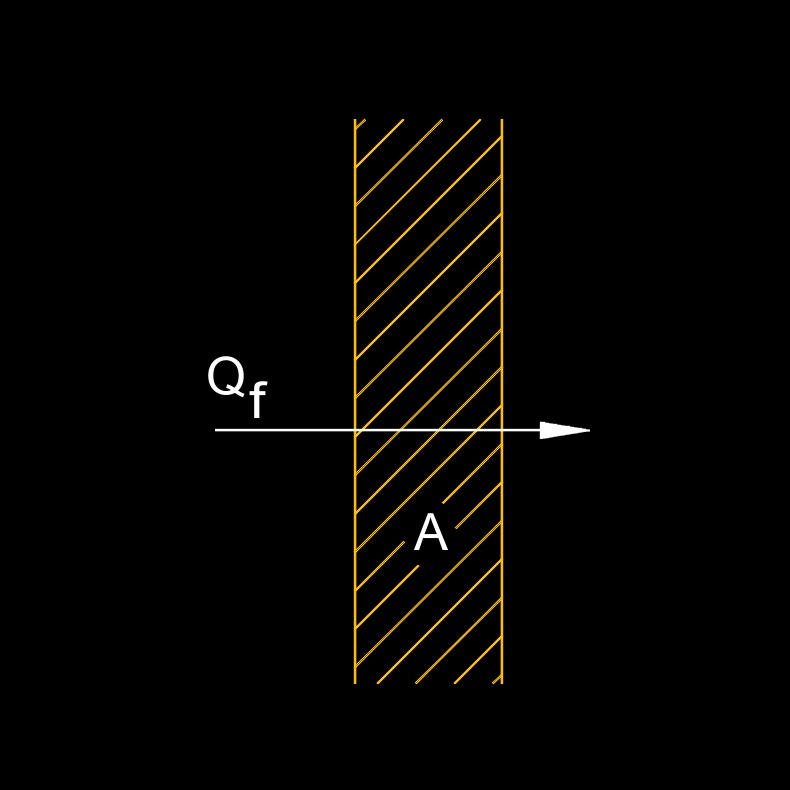 Heat Flux - The heat flow rate transfer through a given surface, per unit surface.
Heat Flux - The heat flow rate transfer through a given surface, per unit surface.
- Heat Loss - The measure of the total heat transfer through your body, clorhing, building walls, etc. This loss happens in four different ways to transfer heat: conduction, convection, mixing, and radiation.
- Heat of Vaporization - The amount of heat needed to be consumed to vaporize a specific amount of liquid at a constant temperature.
- Heat Penetration - The depth at which heat from any heat source dissipates into a source.
- Heat Radiation - See Heat Transfer by Radiation
- Heat Transfer - The exertion of power that is created by heat, or the increase in temperature. It is the transfer of heat from one system to another. There are four ways to transfer heat: conduction, convection, mixing, and radiation.
- Heat Transfer by Conduction - It is the flow of energy between two objects, or within one object, where there is a temperature differential.
- Heat Transfer by Convection - Energy transferred by random molecular motion as well as energy transferred by the bulk motion of the fluid.
- Heat transfer by Radiation - Radiation is the flow of energy through electromagnetic waves such as infrared, light, microwaves, etc.
- Heat Transfer Coefficient - The convective heat transfer between a solid surface and the fluid around it.
- Heat Transfer Coefficient of a Pipe Wall - The resistance to the flow of heat by the pipe wall material can be expressed by the heat transfer coefficient of the pipe wall.
- Heat Transfer Rate - The amount of hear transfered per unit of time per fluid or material.
- Henry's Law - One of the gas laws, at a constant temperature, the volume of a gas which will dissolve into a solution is directly porportional to the partial pressure of that gas above the solution in equilibrium with the liquid.
- High Pressure Steam - When the pressure greatly exceeds that of the atmosphere.
I
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Ideal Gas - Defined as one in which all collisions between atoms or molecules are perfectly elastic and in which there are no intermolecular attractive forces.
- Ideal Gas Law - Used to predict pressure, temperature & volume changes in ideal gasses.
- Ideal Gas Law with Compressibility Factor - Used for higher pressure and temperature than the ideal gas law that is at atmospheric conditions.
- Ignition Temperature - The minimum temperature to which a fuel must be brought to start the combustion.
- Inert Gas - A gas which does not normally combine chemically with the base metal or filler metal.
- Internal Energy - The total of all energies associated with the motion of the molecules in the system.
- Intensive Properties - See intrinsic properties.
- Intrinsic Properties - Those properties which are independent of the mass of the system, such as density, pressure, and temperature.
- Isobaric Process - The pressure is kept constant. To do this, the volume needs to expand or contract in a way that any pressure change would be negated that would be caused by heat transfer in the system.
-
Isobaric Process - Enthalpy - A thermodynamic process where the pressure is kept constant, \(\Delta p = 0\).
-
Isobaric Process - Entropy - A thermodynamic process where the pressure is kept constant, \(\Delta p = 0\).
- Isobaric Process - Entropy in Terms of Pressure and Volume - Isobaric process is a thermodynamic process where the pressure is kept constant, \(\Delta p = 0\).
- Isolated System - Keeps the energy and matter within the system and everything else out. No transfer in or out.
- Isothermal Process - An isothermal process is a process that happens when there are no variations (constant) in the temperature of the system, but other parameters such as volume and pressure, can be changed accordingly.
J
K
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Kelvin - A unit of temperature normally used for scientific calculations.
- Kinetic Energy - The energy in moving objects or mass. If it moves, it has kinetic energy.
L
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Latent Energy - The internal energy associated with the phase of a system.
- Latent Heat - The energy absorbed or released by a substance during a constant temperature or phase change from a solid to liquid, liquid to gas or vise versa.
- Linear Thermal Expansion - A porportional change in the origional length and change in temperature due to the heating or cooling of an object.
- Linear Thermal Expansion Coefficient - The ratio of the change in size of a material to its change in temperature.
- Liquid Vapor Mixture - The same substance can coexist at the same pressure and temperature.
- Logarithmic Mean Temperature Differential - Used to determine the temperature driving force for heat transfer usually in heat exchangers.
- Log Mean Temperature Differential - See Logarithmic Mean Temperature Differential
- Low Pressure Steam - When the pressure is less than or equal to that of the atmosphere.
M
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Mass Fraction - The ratio of the mass of one component in a mixture to the total mass of the minture.
- Matter - When you look around, everything you see or may not see in the universe is made up of matter. If it has a mass and takes up space, it is matter.
- Melting Point - A solid is the temperature at which it changes state from solid to liquid.
- Modulus of Elasticity - See Young's Modulus
- Molecular Mass - See Molecular Weight
- Molecular Weight - The average mass of one molecule of a substance, 1⁄12 (0.083) the mass of carbon 12 atom.
N
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Newton's Law of Cooling - The law states that the rate of heat loss of a body is directly porportional to the difference in the temperature between the body and its surrounding provided the temperature difference is small and the nature of radiating surface remains the same.
O
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Open System - Freely exchanges energy and matter with its surroundings.
- Open, Closed, and Isolated Systems - A thermodynamics system is a specific quanity of matter with a defined boundary and everything outside the boundary is the surroundings. The system boundary can either be stationary or moving. There are three types of thermodynamics systems: open, closed, and isolated.
- Overall Heat Transfer Coefficient - The heat transfer between items like walls in buildings or across heat exchangers for the conduction within materials.
P
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Peng-Robinson Equation of State - Developed in 1976 at the University of Alberta in order to satisfy 4 goals.
- Plant Steam - The steam generated in an industrial or commercial facility for various applications within the plant.
- Pressure of an Ideal Gas - In an ideal gas, molecules have no volume and do not interact.
Q
R
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Radiant Energy - Is transmitted without the movement of mass.
- Radiant Heat - Heat communicated by radiation and transmitted by electromagnetic waves.
- Radiation Heat Transfer - See Heat Transfer by Radiation
- Rankine - Aunit of temperature used in the US engineering field. 0 is set at absolute zero.
- Redlich-Kwong Equation of State - Derives from the Van der Waals equation and generally more accurate.
S
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Saturation Temperature - Temperature of the liquid surface corresponding to the pressure of the vapour in contact with in.
- Saturated Phase - Any phase of a substance existing under saturated conditions, wherein two or more phases of a pure substance can exist togeather in equilibrium.
- Saturated Steam - The point (temperature and pressure) when steam is in contact with the liquid water (boiling) it came from.
- Saturated Vapor - A vapor at a temperature of the boiling point on the verge of condensing.
- Second Law of Thermodynamics - States that heat cannot pass from a cold body to a hot one by a self-acting process without the help of an external influence.
- Semi-perfect Heat - One which follows the ideal gas relations with specific heats being functions of temperature.
- Sensible Heat - The heat added to a substance which increases its temperature but not the phase.
- Shrinkage - The contraction in volume as a liquid or metal cools or freezes from its normal temperature.
- Solubility - The maximum amount of solid that can be dissolved in a liquid at a specific temperature.
- Specific Enthalpy - The enthalpy per unit mass. It is also the total specific internal energy in a system and the product of pressure and specific volume.
- Specific Internal Energy - The internal energy per unit mass of a substance.
- Specific Heat - The amount of heat required to raise the temperature of a material 1 degree.
- Specific Heat Capacity - The amount of energy required to increase the temperature of the substance by 1°C.
- Steam - The invisible vapor (gas) when water is heated to its boiling point and passes from a liquid to a gaseous state.
- Steam density - Has a higher density than water vapor, the higher the pressure the higher the steam density.
- Steam Dryness - See Steam Quality
- Steam Flow Regimes - Because steam can behave as a liquid and as a gas and everything inbetween, the way steam flows through a pipe can change through long straight runs.
- Steam Quality - The porportion of saturated steam (vapor) in a saturated condensate (liquid) / steam (vapor) mixture.
- Steam Users - Steam is used for many things in piping design. In the oil and gas industry, steam is used to heat production tanks, keep flow lines from freezing and to increase oil production in formations that are very viscious.
- Sublimation - A physical change of a substance from a solid phase to a gas phase and does not pass through the transitional liquid phase.
- Subcooled Liquid - A liquid existing at a temperature lower than its saturated temperature, or at a pressure higher than its saturated pressure.
- Superheat - Addition of heat to a fluid after it has completely vaporized. The temperature increases but pressure does not.
- Superheated Steam - Steam at any given pressure which is heated to a temperature higher than the temperature of saturated steam.
- Superheated Vapor - When vapor has absorbed more heat than is needed to vaporize. It will not condense when small amounts of heat is removed.
T
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Temperature - The amount of heat or cold, but it is neither heat or cold. Temperature is expressed as a number that is related to energy and porportional to a type of energy, but it is not energy.
- Temperature Differential - The difference between two specific temperature points of a volume at a given time in a system.
- Temperature Gradient - Describes in which direction and what rate the temperature changes in a given area.
- Temperature of an Ideal Gas - In an ideal gas, molecules have no volume and do not interact.
- Tension Modulus - See Young's Modulus
-
Thermal - Caused by the change in temperature.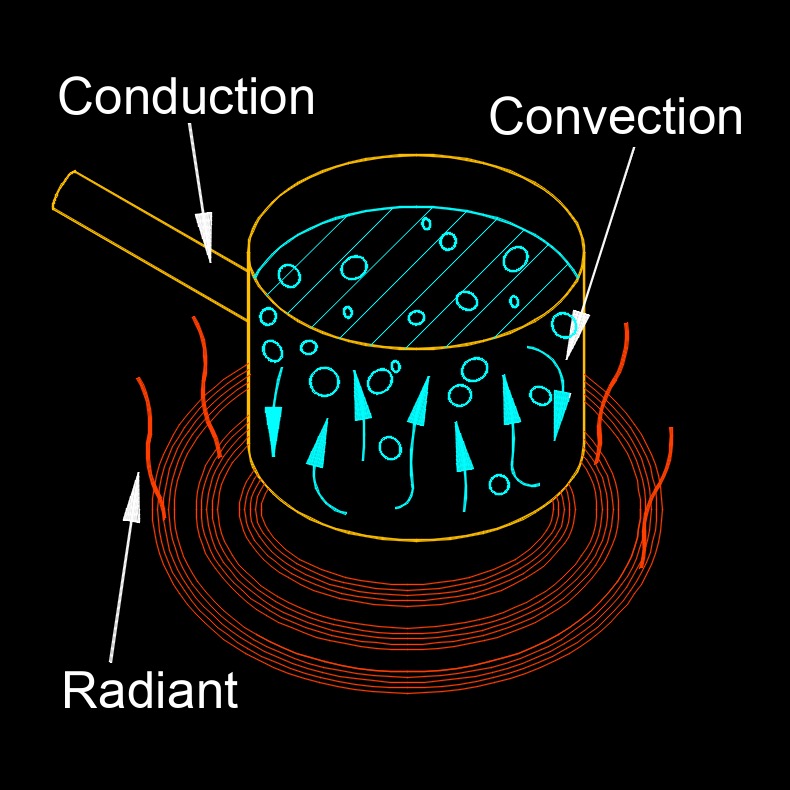
- Thermal Analysis - A method of studying transformations in metal by measuring the temperature at which thermal arrests occure.
- Thermal Capacity - See Heat Capacity
- Thermal Conductivity - The ability to transfer heat within a material without any motion of the material.
- Thermal Contraction - The decrease in a linear dimension and volume of a material with a change of temperature.
- Thermal Cracking - A fluid degradation that occurs when a fluid is heated above the maximum bulk temperature. Consequently, the fluid's viscosity, flash point, fire point and auto-ignition temperature reduce significantly.
- Thermal Diffusivity - A measure of the transient thermal reaction of a material to a change in temperature.
- Thermal Efficiency - The fraction of heat that is converted to work or desired output divided by required input.
- Thermal Energy - The exertion of power that is created by heat, or the increase in temperature.
- Thermal Expansion - The increase in length, area or volume due to the increase (in some cased decrease) in temperature.
- Thermal Expansion Coefficient - The percentage change in the length of the material per degree of temperature change, heated solid or liquid.
- Thermal Fatique - Failure resulting from rapid cycles of alternating heating and cooling.
- Thermal Flux - See Heat Flux
- Thermal Insulation - Any material that is used as a barrier to heat transfer.
- Thermal Insulator - Does not conduct heat readily and is used for either heat conservation or personnel protection.
-
Thermal Resistance - Measures the temperature difference by which an object or material resists a heat flow.
- Thermal Stability - The ability of a fluid to resist degradation under high-temperature operating conditions.
- Thermal Stress - Results from non uniform distribution of temperature.
- Third Law of Thermodynamics - Is about the ability to create an absolute zero temperature, where the entropy approaches a constant minimum value. It also states that it is not possible for any system to reach absolute zero.
- Total Energy - The sum of numerous forms of energy such as chemical, electrical, magnetic, mechanical, nuclear, potential, thermal, and etc.
- Total Heat Transfer - The heat put into a system or heat lost from a system.
U
V
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Van der Waals Equation - A modification of the ideal gas law, corrects for the volume of, and attractive forces between gas molecules.
- Volume Differential - The difference between an expanded or reduced volume of a liquid.
-
Volumetric Thermal Expansion - Takes place in gasses and liquids when a change in temperature, volume or type of substance occures.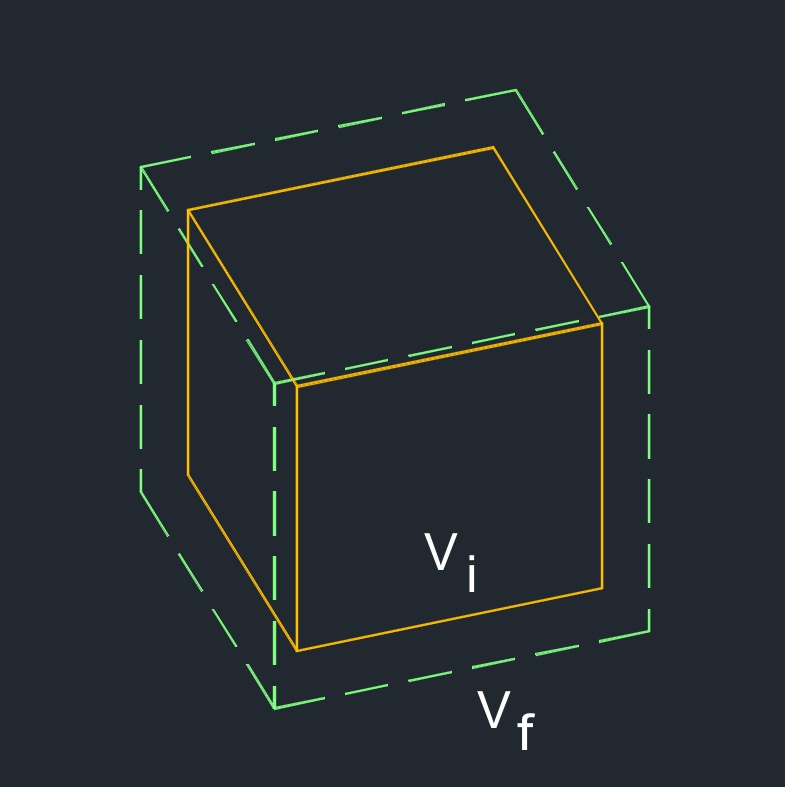
- Volumetric Thermal Expansion Coefficient - The ratio of the change in size of a material to its change in temperature.
W
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Waste Heat - Energy that must be dissipated to the atmosphere from a process such as the heat transfer from condensing steam in the condenser of a system power plant.
- Wet Steam - Wet steam contains both water and steam held in suspension just below the satutation temperature.
- Work - The overcoming of resistance through space and is the measure of force x distance.
X
Y
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Young's Modulus - Measures the stiffness of an elastic material. The ratio of the longitudinal stress applied to a body or substance to the resulting longitudinal strain within the elastic limits.
Z
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Zeroth Law of Thermodynamics - When two thermal systems are in equilibrium and they with a third, then all are equal to each other, meaning \(A=B\) and \(B=C\) then \(A=C\)

Tags: Physics

