Laws of Thermodynamics
Laws of Thermodynamics Index
- First Law of Thermodynamics
- Second Law of Thermodynamics
- Third Law of Thermodynamics
- Zeroth Law of Thermodynamics
First Law of Thermodynamics
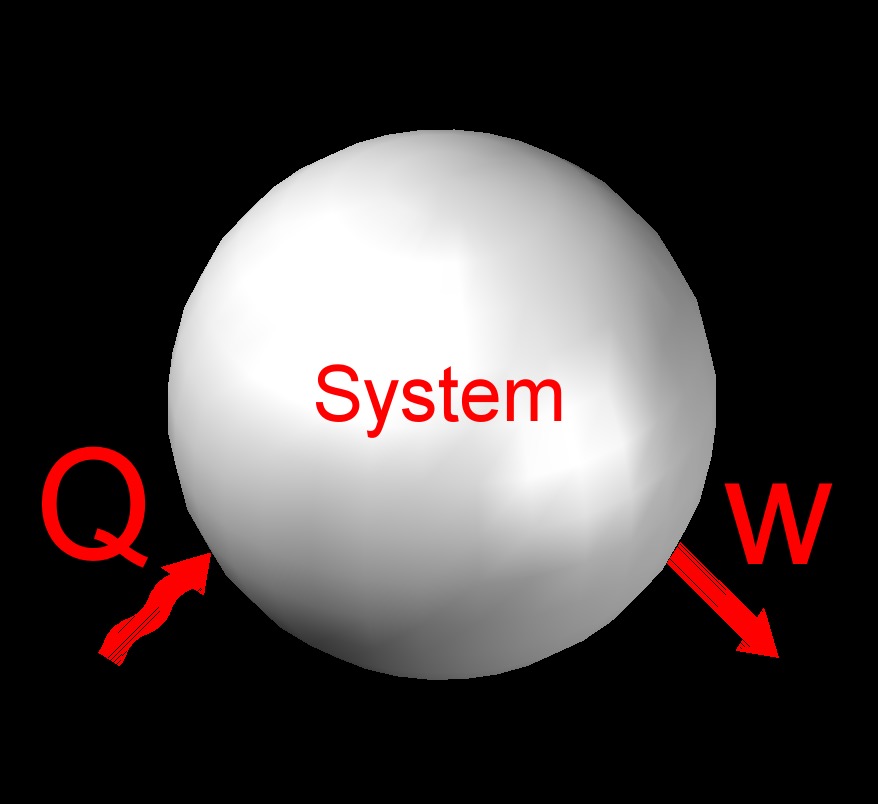 The first law of thermodynamics, also called conservation of energy, expresses the principal of the conservation of energy. This means that the total amount of energy in the universe is constant and that it can neither be created or destroyed. This law states that for every gain in some type of energy will result in the loss in some other form.
The first law of thermodynamics, also called conservation of energy, expresses the principal of the conservation of energy. This means that the total amount of energy in the universe is constant and that it can neither be created or destroyed. This law states that for every gain in some type of energy will result in the loss in some other form.
In general, the conservation of energy can be stated as follows:
Kinetic Energy + Potential Energy = Constant
First Law of Thermodynamics formula |
||
|
\( \Delta U \;=\; Q - W \) (First Law of Thermodynamics) \( Q \;=\; \Delta U + W \) \( W \;=\; Q - \Delta Q \) |
||
| Symbol | English | Metric |
| \( \triangle U \) = internal energy | \(lbf-ft\) | \(J\) |
| \( Q \) = heat added to the system | \(F\) | \(C\) |
| \( W \) = work done by the system | \(lbf-ft\) | \(J\) |
Second Law of Thermodynamics
The second law of thermodynamics, also called entropy, abbreviated as S, is a fundamental principle in physics and thermodynamics that describes the behavior of energy in a system. It can be stated in multiple ways, but one common formulation is, "The total entropy of an isolated system always tends to increase over time." Entropy can be thought of as a measure of the disorder or randomness in a system. The law implies that in any natural process, the overall level of disorder in the universe, as measured by entropy, will tend to increase or remain the same.
Another way to state the second law is that heat flows spontaneously from a hotter body to a colder body, but not in the reverse direction without external intervention. This is known as the principle of the thermal gradient or the Clausius statement of the Second Law. This law also leads to the concept of energy degradation or dissipation. In any energy transformation or conversion, such as the conversion of heat energy into mechanical work, some energy will inevitably be lost or dissipated as waste heat, which increases the entropy of the system and the surroundings.
It is important to note that while the second law of thermodynamics indicates a tendency towards increasing entropy and energy degradation, it does not imply that isolated systems cannot experience temporary decreases in entropy or localized decreases in entropy at the expense of greater increases elsewhere. However, the net change in entropy for the entire system and its surroundings will always be positive or zero.
Overall, the second law of thermodynamics has wide ranging applications and implications, from understanding the efficiency limits of heat engines to explaining natural phenomena such as diffusion, irreversibility, and the arrow of time.
Second Law of Thermodynamics formula |
||
|
\( \Delta S \;=\; \Delta Q \;/\; T \) (Second Law of Thermodynamics) \( \Delta Q \;=\; \Delta S \; T \) \( T \;=\; \Delta Q \;/\; \Delta S \) |
||
| Symbol | English | Metric |
| \( \Delta S \) = entropy differential | \(Btu\;/\;lbm-R\) | \(kJ\;/\;kg-K\) |
| \( \Delta Q \) = heat differential (energy) | \(Btu\;/\;lbm\) | \(J\;/\;kg\) |
| \( T \) = temperature | \(R\) | \(K\) |
Third Law of Thermodynamics
The third law of thermodynamics, also known as Nernst's theorem or the Nernst heat theorem, is about the ability to create an absolute zero temperature, where the entropy approaches a constant minimum value. It also states that it is not possible for any system to reach absolute zero. More specifically, the third law of thermodynamics states that the entropy of a pure, perfect crystal at absolute zero temperature is exactly equal to zero. In other words, as the temperature approaches absolute zero, the entropy of a perfect crystal approaches zero.
Entropy is a measure of the disorder or randomness in a system, and the third law implies that at absolute zero, a perfect crystal would have no randomness or disorder. While it is practically impossible to reach absolute zero in reality, this law provides a theoretical foundation and boundary condition for studying the behavior of systems at extremely low temperatures. This law has significant implications for fields such as condensed matter physics and the study of phase transitions, where the behavior of materials at very low temperatures is of interest. It also helps in understanding phenomena like superconductivity and superfluidity that occur at extremely low temperatures.
Zeroth Law of Thermodynamics
The zeroth law of thermodynamics is one of the fundamental principles in thermodynamics. When two thermal systems are in equilibrium and they with a third, then all are equal to each other, meaning \(A=B\) and \(B=C\) then \(A=C\). If a system does not transfer heat, it is in thermal equilibrium even though it can transfer heat. It establishes the concept of temperature and thermal equilibrium.
In simpler terms, it means that if two objects A and B are separately in thermal equilibrium with a third object C, then A and B will be in thermal equilibrium with each other when brought into contact, meaning they will have the same temperature. This principle allows us to define and measure temperature and enables the construction of reliable thermometers.
The Zeroth Law is called "zeroth" because it was added after the first and second laws of thermodynamics had already been established. It is considered "zeroth" in order to preserve the logical sequence of the laws.

Tags: Temperature Energy Laws of Physics Laws of Thermodynamics

