Cathodic Protection
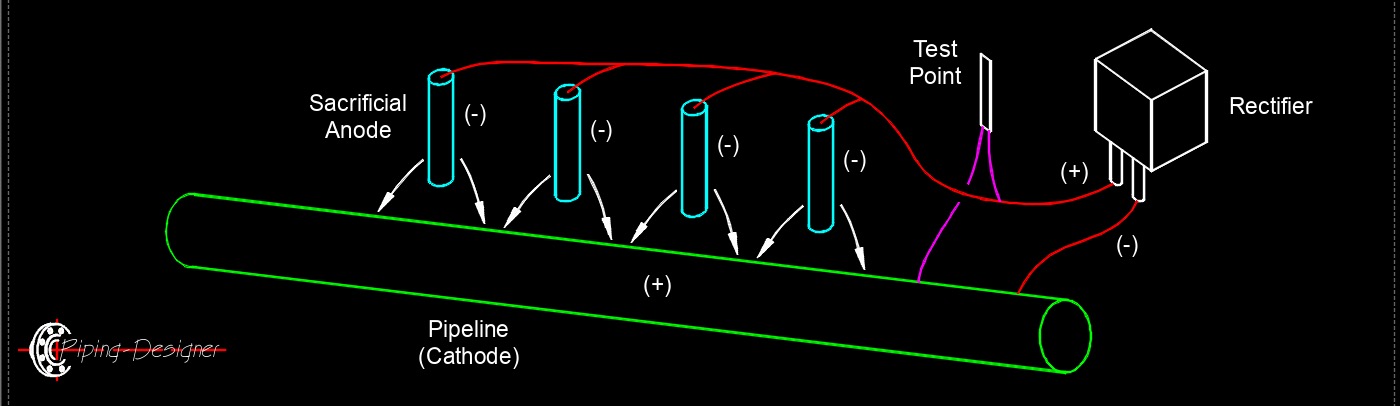 Cathodic protection, abbreviated as CP, is a technique used to prevent corrosion of metal structures and equipment by making them the cathode of an electrochemical cell. Corrosion occurs when metal is exposed to an electrolyte, such as water or soil, and a flow of electrons occurs between the metal and the electrolyte, resulting in the metal corroding. By making the metal structure the cathode of an electrochemical cell, the flow of electrons is reversed, and corrosion is prevented. CP is typically achieved by connecting a sacrificial anode made of a more active metal, such as zinc or magnesium, to the metal structure being protected. The sacrificial anode corrodes instead of the metal structure, providing cathodic protection. Another method of cathodic protection is impressed current cathodic protection, which involves using a direct current power source to provide the required current flow to the structure being protected.
Cathodic protection, abbreviated as CP, is a technique used to prevent corrosion of metal structures and equipment by making them the cathode of an electrochemical cell. Corrosion occurs when metal is exposed to an electrolyte, such as water or soil, and a flow of electrons occurs between the metal and the electrolyte, resulting in the metal corroding. By making the metal structure the cathode of an electrochemical cell, the flow of electrons is reversed, and corrosion is prevented. CP is typically achieved by connecting a sacrificial anode made of a more active metal, such as zinc or magnesium, to the metal structure being protected. The sacrificial anode corrodes instead of the metal structure, providing cathodic protection. Another method of cathodic protection is impressed current cathodic protection, which involves using a direct current power source to provide the required current flow to the structure being protected.
Cathodic Protection Index
- Cathodic Protection Types
- Cathodic Protection Advantages and Disadvantages
- Cathodic Protection Standards
- Cathodic Protection Glossary
CP is commonly used in a variety of industries, including oil and gas, marine, and transportation, to protect metal structures such as pipelines, tanks, and ships from corrosion. It is a highly effective technique for preventing corrosion, and can extend the life of metal structures significantly. CP systems require regular monitoring and maintenance to ensure that they continue to function properly over time. Corrosion engineers and technicians are responsible for designing, installing, and maintaining cathodic protection systems to ensure that metal structures remain protected from corrosion.
Science Branches |
| Science |
| Applied Science |
| Engineering |
| Chemical Engineering |
|
Cathodic Protection Types
- Galvanic anode CP (GACP) - Also called sacrifical CP. Uses a sacrifical anode that corrodes before the material being protected does by an electrochemical reaction (no power source needed).
- Impressed current CP (ICCP) - Uses a sacrificial anode connected to an external DC power source. DC flows from source to anode, to protected material, to source.
- Impressed current anode material - graphite, high silicon cast iron, and mixed metal oxide
- Impressed current anode pro
- Current can be controlled, with no limit of driving voltage
- Can be remotely controlled
- Can replace anodes when needed
- Impressed current anode con
- Require regularly monitoring or maintenance
- Requires power source
- More monitoring or maintenance means more likely the breakdowns.
Cathodic Protection standards
ASME Standards
- ASME B16.5 - Pipe Flanges and Flanged Fittings: NPS 1/2 through NPS 24 Metric/Inch Standard
- ASME B16.9 - Factory-Made Wrought Buttwelding Fittings
- ASME B16.47 - Large Diameter Steel Flanges, NPS 26 Through NPS 60
- ASME B16.36 - Orifice Flanges
- ASME B31.4 - Pipeline Transportation Systems for Liquid Hydrocarbons and Other Liquids
- ASME B31.8 - Gas Transmission and Distribution Piping Systems
- ASME G8 - Standard Test Methods for Cathodic Disbonding of Pipeline Coatings
- ASME G14 - Testing Method for Impact Resistance of Pipeline Coatings (Falling Weight Test)
- ASME G95 - Testing Method for Cathodic Disbonding of Pipeline Coatings (Attached Cell Method)
AWWA Standards
- AWWA C209 - Cold-Applied Tape Coating for the Exterior of Special Sections, Connections, and Fittings for Steel Water Pipelinss
- AWWA C214 - Tape Coating Systems for the Exterior of Steel Water Pipelines
- AWWA C216 - Heat-Shrinkable Cross-Linked Polyolefin Coatings for the Exterior of Special Sections, Connections, and Fittings for Steel Water Pipelinss
- AWWA C222 - Polyurethane Coatings for the Interior and Exterior of Steel Water Pipe and Fittings
NACE Standards
- NACE RP0186 - Application of Cathodic Protection for External Structures of Steel Well Casings
- NACE SP0102 - In-Line Inspection of Pipelines
- NACE SP0104 - The Use of Coupons for Cathodic Protection Monitoring Applications
- NACE SP0106 - Control of Internal Corrosion in Steel Pipelines and Piping Systems
- NACE SP0169 - Control of External Corrosion on Underground or Submerged Metallic Piping Systems
- NACE SP0188 - Discontinuity (Holiday) Testing of Protective Coating
- NACE SP0193 - External Cathodic Protection of On-Grade Carbon Steel Storage Tank Bottoms
- NACE SP0216 - Steel-Cased Pipeline Practices
- NACE SP0205 - External Corrosion Control of Underground Storage Tank Systems by Cathodic Protection
- NACE SP0207 - Close-Internal Potential Surveys on Buried or Submerged Metalic Pipelines
- NACE SP0216 - Sacrificial Cathodic Protection of Reinforcing Steel in Atmospherically Exposed Concrete Structures
- NACE SP0290 - Impressed Current Protection of Reinforcing Steel in Atmospherically Exposed Concrete Structures
- NACE SP0502 - Pipeline External Corrosion Direct Assessment Methodology
- NACE SP0607 - Petroleum and Natural Gas Industries-Cathodic Protection of Pipeline
- NACE SP21434 - Cathodic Protection Systems for the Mitigation of External Corrosion of Buried and Submerged Metallic Piping Systems at Nuclear Power Plants
- NACE Publication 1E100 - Engineering Symbols Related to Cathodic Protection
- NACE TR01105 - Sacrificial Cathodic Protection of Reinforced Concrete Elements
- NACE TR21447 - Consequences of Coating Failures as Related to Interaction with Cathodic Protection
- NACE TR21463 - Criteria for Evaluation of Cathodic Protection Methods for Steel in Existing Concrete Structures
Tags: Abbreviations Nomenclature and Symbols
Cathodic Protection Glossary
A
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Abradable Coating - It gives wear resistance to highly abrasive material when rubbed against, while leaving the underlying material damage free.
- Acid - A chemical substance that yields hydrogen ions when dissolved in water.
- Acid Embrittlement - A form of hydrogen embrittlement that may be introduced in some metals by acid.
- Activation - The changing of a passive surface of a metal to a chemically active state.
- Active Metal - A metal ready to corrode, of being corroded.
- Active Potential - The potential of a corroding material.
- Addition Agent - A substance added to a solution for the purpose of altering or controlling a process.
- Alclad - Composit wrought product comprised of an aluminum alloy core having on one or both surfaces a metallurgically bonded aluminum or aluminum alloy coating that is anodic to the core and thus electrochemically protects the core against corrosion.
- Alternating Current - An electric current that reverses its direction over and over.
- Anchoride - A zinc-iron phosphate coating for iron and steel.
- Anion - A negatively charged ion.
- Anode - The electrons flow away the anode (negative charge) at which corrosion or oxidation occures at the material.
- Anode Corrosion - The dissolution of a metal acting as an anode.
- Anode Corrosion Efficiency - The ratio of actual to theoretical corrosion based on the total current flow calculated by Faraday's law from the quantity of electricity that has passed.
- Anodic Coating - A film on a metal surface resulting from an electrolytic treatment at the anode.
- Anodic Inhibitor - A chemical substance or combination of substances that prevent or reruce the rate of the anodic or oxidation reaction by a physical, physico-chemical or chemical action.
- Anodic Polarisation - The electrochemical state changing of an electrode's potential moving in a corroding positive direction.
- Anodic Potential - An appreciable reduction in corrosion by making a metal an anode and maintaining this highly ploarized condition with very little current flow.
- Anodic Reaction - Electrode reaction equivalent to a transfer of positive charge from the electronic to the ionic conductor. An anodic reaction is an oxidation process.
- Anodizing - Forming a conversion coating on a metal surface by anodic oxidation; most frequently applied to aluminum.
- Atmospheric Corrosion - A gradual degradation of alteration of a material by contact with substances present in the atmosphere, such as oxygen, carbon dioxide, water vapor, sulfur and chlorine compounds.
- Auxiliary Anode - A supplementary anode positioned so as to raise the current density on a certain area of the catnode and thus obtain distribution of plating.
- Auxiliary Electrode - An electrode commonly used in polorization studies to pass current to or from a test electrode, usually made of noncorroding material.
B
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Backfill - Material placed in a drilled hole to fill space around anodes amd buried components.
- Back Ionisation - A condition, which may occur during electrostatic application of powder coating where excessive build up of charged powder particles limits further powder to be deposited onto the substrate.
- Bainite - A metastable aggregate of ferrite and cementite resulting from the transformation of austenite at temperatures below the pearlite range but above the martensite start temperature.
- Barrier Coating - A protective layer of material that prevents the contact of corrosive elements.
- Base - A substance that releases hydroxyl ions when disolved in water.
- Black Liquor - The liquid material remaining from pulpwood cooking in the soda or sulfate paper-making process.
- Black Oxide - A black finish on a metal produced by immersing it in hot oxidizing salts or salt solutions.
- Blast Cleaning - Removal of surface contamination and corrosion products by use of air and mechanical abrasives.
- Blistering Bubbles - Formed under the cured powder film, usually caused by the expansion of trapped air, moisture or corrosion.
- Blooming - A hazy appearance on the surface of a coating.
- Blushing - Whithering and loss of gloss of a usually organic coating caused by moisture.
- Brine - Seawater containing a higher concentration of dissolved salt than that of the ordinary ocean.
C
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Cathode - The electrons flow toward the cathode reducing the corrosion or oxidation of the material.
- Cathode Cleaning - Electrolytic cleaning in which the work is the cathode.
- Cathode Corrosion - Corrosion resulting from a cathodic condition of a structure usually caused by the reaction of an amphoteric metal with the alkaline products of electrolysis.
- Cathode Disbondment - The destruction of adhesion between a coating and its substrate by products of a cathodic reaction.
- Cathode Efficiency - Current efficiency at the cathode.
- Cathode Film - The portion of solution in immediate contact with the cathode during electrolysis.
- Cathodic Pickling - Electrolytic pickling in which the work is the cathode.
- Cathodic Polarisation - The electrochemical state changing of an electrode's potential moving in a non-corroding negative direction.
- Cathodic Reaction - Electrode reaction equipment to a transfer of negative charge from the electrode to the ionic conductor.
- Catholyte - The electrolyte adjustment to the cathode of an electrolytic cell.
- Catholyte - A positively charged ion that migrates through the electrolyte toward the cathode under the influence of a potential gradient.
- Corrosion - The thinning of a pipe wall that is typically caused by a chemical reaction from a corroding fluid or agent and is limited almost exclusively to metal products.
- Corrosion Allowance - The amount of material in a pipe or vessel that is available for corrosion without affecting the pressure containing integrity.
- Corrosion Coupon - Used to monitor the corrosion rate of a material in a process.
- Corrosion Inhibitor - A substance that slows down the chemical reaction rate of corrosion on metal that is exposed to the environment.
- Corrosion Mapping - An ultrasonic method that identifies and maps corroded areas in a pipeline by the varying material thickness.
- Crack - Cracks can come from fatigue, grith welds, or seam welds.
- Current - The rate of flow of electricity in a circuit, measured in amperes.
- Current Density - The measure of current per unit of cross section.
- Current Efficiency - The ratio of the electrochemical equivalent current density for a specific reaction to the total applied current density.
D
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Depolarization - When the CP cirrent stops flowing from athe anode to the structure being protected, the polarized structure will then begin to depolarize.
- Descaling - Removing the thick layer of oxides formed on some metals at elevated temperatures.
- Dezincification - Corrosion at which zinc is selectively leached from zinc-containing alloys.
- Diffusion - The spread of gases, liquids, or solids from areas of high concentration to areas of low concentration.
- Diffusion Coefficient - Expresses the transfer rate of a substance by random molecular motion.
- Direct Current - An electric current that flows in only one direction.
- Dummy Cathode - A substitute cathode that is used during adjustment of operating conditions.
E
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Electric Conductivity - The amount of current that a material can conduct.
- Electric Current Density - The measure of current per unit of cross section.
- Electrochemical Cell - A system consisting of an anode and a cathode in metallic contacts and immersed in an electrolyte.
- Electrochemical Corrosion - Corrosion that is accompanied by a flow of electrons between cathodic and anodic areas on metallic surfaces.
- Electrode - Refered to as the anode or cathode, whichever is approperate.
- Electrode Potential - The potential of an electrode in an electrolyte as a measure against a reference electrode.
- Electrolyte - A chemical substance or mixture, liquid or solid, normally liquid, which conducts electric current.
- Etching - Removal of a layer of the base metal.
- External Corrosion - When the outside of a pipe is decayed or eroded by chemical or electrochemical processes or any other environmental conditions.
F
- Faraday's Law of Induction - States that whenever a conductor is placed in a varying magnetic field, an electromotive force is introduced.
- Fatigue - The application and release of stresses as metal is used which cause small cracks to grow, during many cycles of application, until they fracture.
- Fish Eyes - Areas on a steel fracture surface having a characteristic white crystalline appearance.
- Flakes - Short, discontinuous internal fissures in wrought metals attributed to stresses produced by localized transformation and decreased solubility of hydrogen during cooling after hot working.
- Flux - Chemicals used to protect metals from oxidation.
- Free Carbon - The part of the total carbon in steel or cast iron that is present in elemental form as graphite or temper carbon.
- Free Corrosion Potential - Corrosion potential in the absence of net electrical current flowing to or from the metal surface.
- Fetting - A type of wear that occurs between tight-fitting surfaces subjected to cyclic relative motion of extremely small amplitude.
- Fretting Corrosion - Takes place where there is friction between two metal surfaces.
G
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Galvanic - Pertaining to the current resulting from the coupling of dissimilar electrodes in an electrolyte.
- Galvanic Anode - A metal which because of its relative position in the galvanic series, provides sacrificial protection to metals that are more noble in the series, when coupled in an electrolyte.
- Galvanic Cell - A cell in which chemical change is the source of electrical energy. It usually consists of two dissimilar conductors in contact with each other and with an electrolyte or of two similar conductors in contact with each other and with dissimilar electrolytes.
- Galvanic Corrosion - Accelerated corrosion of a metal because of an electrical contact with a more noble metal or nonmetallic conductor in a corrosive electrolyte.
- Galvanic Current - The electric current that flows between metals or conductive nonmetal in a galvanic couple.
- Galvanize - To coat a metal surface with zinc using any of various processes.
- Gaseous Corrosion - Corrosion with gas as the only corrosive agent and without any aqueous phase on the surface of the metal. Also called dry corrosion.
H
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Hot Corrosion - An accelerated corrosion of metal surfaces that results from the combined effect of oxidation and reactions with sulfur compounds and other contaminants.
- Hot Cracking - Caused by the segregation at grain boundaries of low-melting constituents in the weld metal.
- Hot Dip Coating - A metallic coating obtained by dipping the base metal into a molten metal.
- Hot Working - Deforming metal physically at such a temperature and strain rate that recrystallization takes place simultaneously with the deformation, thus avoiding any strain hardening.
- Humidity Test - A corrosion test involving exposure of specimens at controlled levels of humidity and temperature.
- Hydrogen Disintegration - Deep internal cracks caused by hydrogen.
- Hydrogen Embrittlement - A process resulting in a decrease of the toughness or ductility of a metal due to the presence of atomic hydrogen.
- Hydrogen Overvoltage - Overvoltage associated with the liberation of hydrogen gas.
I
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Impressed Current Anode - They are intended to discharge current when being powered by an external DC power source.
- Incomplete Fusion - A weld break where complete fusion did not occur between the weld material and the faces or adjoining weld material.
- Inhibitor - A chemical substance or combination of substances that, when present in the environment, prevents or reduces corrosion without significant reaction with the components of the environment.
- Internal Oxidation - The formation of isolated particles of corrosion products beneath the metal surface.
- Interupter - A sophisticated switch that can be used to interrult the operation of a rectifier.
- Ion - An atom or molecular particle having a net charge. Positive charged ions are cations and negative charged are anions.
- Ion Exchange - The reversible interchange of ions between a liquid and solid, with no substantial structural changes in the solid.
- Isolation Gasket - Used to stop the current flow across metallic pipelines by separating two flanges.
J
K
L
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Localized Corrosion - Corrosion at discrete sites, stress-corrosion cracking.
M
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Metallic Bonding - The attraction between the metal ions and the free-floating electrons in the lattice structure of metal results in the formation of a metallic bond.
N
O
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Ohm - A unit of resistance.
- Ohm's Law - The relationships between power, voltage, current, and resistance.
- Oxidation - The loss of electrons in a chemical reaction in which an element combines with oxygen. Oxidation and reduction always occur at the same time in equal amounts.
- Oxide - A compound of oxygen with some other chemical elements.
P
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- pH - Affects the corrosion rate by affecting the reaction rate of cathodes and anodes.
- Polarization - When CP current flows from the anode to the structure being protected, that structure's potential will shift more electrically negative. The shift is called polarization.
Q
R
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Rectifier - A power supply that converts AC power to DC power.
- Rust - A corrosion product consisting primarily of hydrated iron oxide.
S
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Stray Current - The flow of electric current into the ground by the leakage on industrial currents.
- Stress - The force per unit area of cross-section.
T
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Tension Strength - The capacity of a material to resist a force tending to stretch it.
U
V
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Vent Pipe - A small diameter pipe that has drilled holes or cut slots that allow gasses generated at the anode during the CP process to vent away from the anode.
- Volt - A unit of electrical pressure.
- Voltage - One volt is the amount of pressure that will cause one ampere of current in one ohm of resistance.
- Voltage Coefficient of Resistance - The change in resistance with applied voltage.
- Voltage Drop - When the voltage at the end of the cable is less than the beginning of the cable.
- Voltage rating - The maximum voltage at which a cable or insulated conductor can be safetly maintained during continuous use in a normal manner.
W
- A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
- Well Integrity - An operation of technical, operational, and organizational solutions to reduce fisk of controlled release of formation fluids throught the life cycle of a well.
- Wetting Agent - A substance that reduces the surface tension of a liquid causing it to spread more readily on a solid.
X
Y
Z

Tags: Cathodic Protection Corrosion

