Nonmetal Element
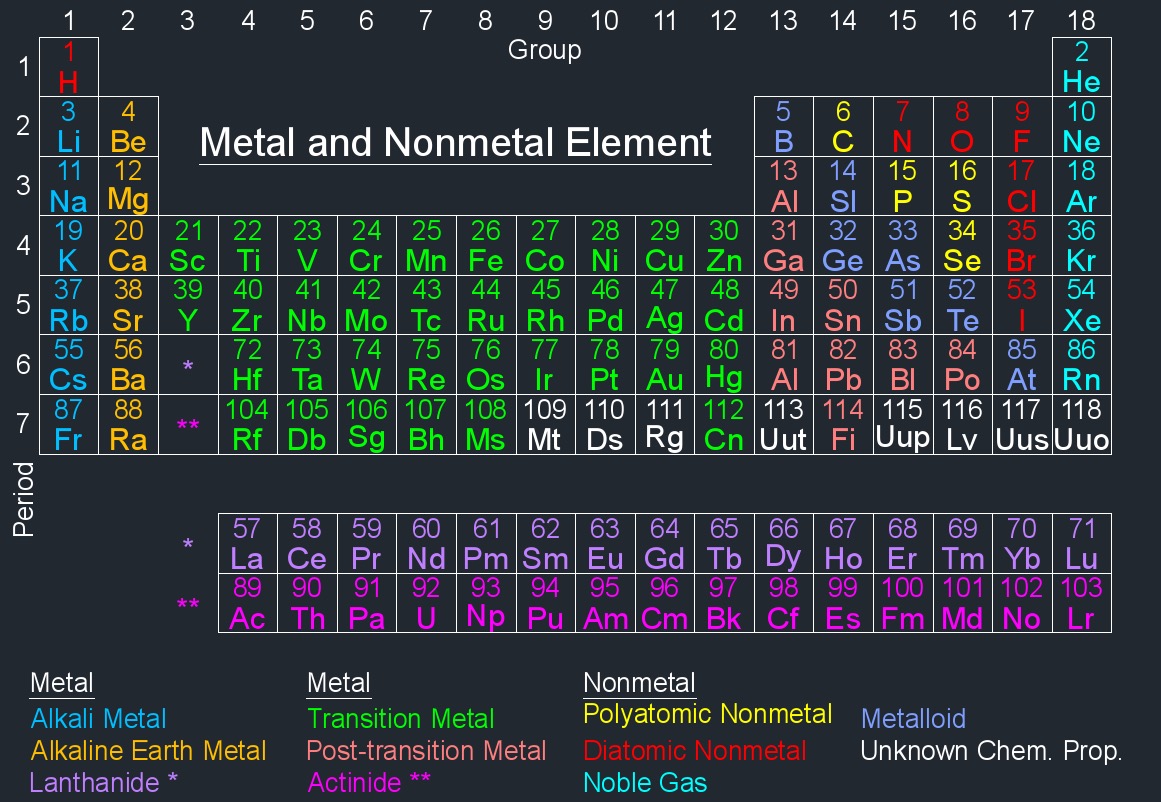 Nonmetal elements are elements that generally lack metallic properties. Unlike metals, nonmetals typically have low melting points and boiling points, are poor conductors of heat and electricity, and tend to have a more diverse range of physical states (solid, liquid, or gas) at room temperature.
Nonmetal elements are elements that generally lack metallic properties. Unlike metals, nonmetals typically have low melting points and boiling points, are poor conductors of heat and electricity, and tend to have a more diverse range of physical states (solid, liquid, or gas) at room temperature.
Diatomic Nonmetal Element | ||||
|---|---|---|---|---|
| Element Name | Element Symbol | Atomic Number | Periodic Group Number | Periodic Period Number |
| Bromine | Br | 35 | 17 | 4 |
| Chlorine | Cl | 17 | 17 | 3 |
| Fluorine | F | 9 | 17 | 2 |
| Hydrogen | H | 1 | 1 | 1 |
| Iodine | I | 53 | 17 | 5 |
| Nitrogen | N | 7 | 15 | 2 |
| Oxygen | O | 8 | 16 | 2 |
A diatomic nonmetal element is a nonmetallic chemical element that exists naturally in the form of diatomic molecules, meaning its atoms bond together in pairs. Diatomic molecules are molecules composed of two atoms of the same element. Many nonmetals form diatomic molecules under normal conditions. These elements are often found in pairs because their atoms are more stable when they share electrons to achieve a full outer electron shell. As a result, in their natural state, they exist as diatomic molecules.
Polyatomic Nonmetal Element | ||||
|---|---|---|---|---|
| Element Name | Element Symbol | Atomic Number | Periodic Group Number | Periodic Period Number |
| Carbon | C | 6 | 14 | 2 |
| Phosphorus | P | 15 | 15 | 3 |
| Selenium | Se | 34 | 16 | 4 |
| Sulfur | S | 16 | 16 | 3 |
A polyatomic nonmetal element refers to a nonmetallic chemical element that typically exists in the form of polyatomic ions or molecules. Polyatomic means that the element forms molecules or ions composed of more than two atoms.
Noble Gas Element | ||||
|---|---|---|---|---|
| Element Name | Element Symbol | Atomic Number | Periodic Group Number | Periodic Period Number |
| Argon | Ar | 18 | 18 | 3 |
| Helium | He | 2 | 18 | 1 |
| Krypton | Kr | 36 | 18 | 4 |
| Neon | Ne | 10 | 18 | 2 |
| Radon | Rn | 86 | 18 | 6 |
| Ununoctium | Uuo | 118 | 18 | 7 |
| Xenon | Xe | 54 | 18 | 5 |
Noble gases (Inert Gas) until the 1960's were considered inert gases. There are seven noble gases all found in Group 18 (VIIIa) in the periodic table and are colourless, odourless, tasteless and nonflammable. They are also known as inert gases because they were once believed to be chemically inert, meaning they do not easily react with other elements. Noble gases have complete electron shells, which makes them stable and less likely to form chemical bonds with other elements. This stability is a result of having a full complement of electrons in their outermost energy levels.

