Metal Element
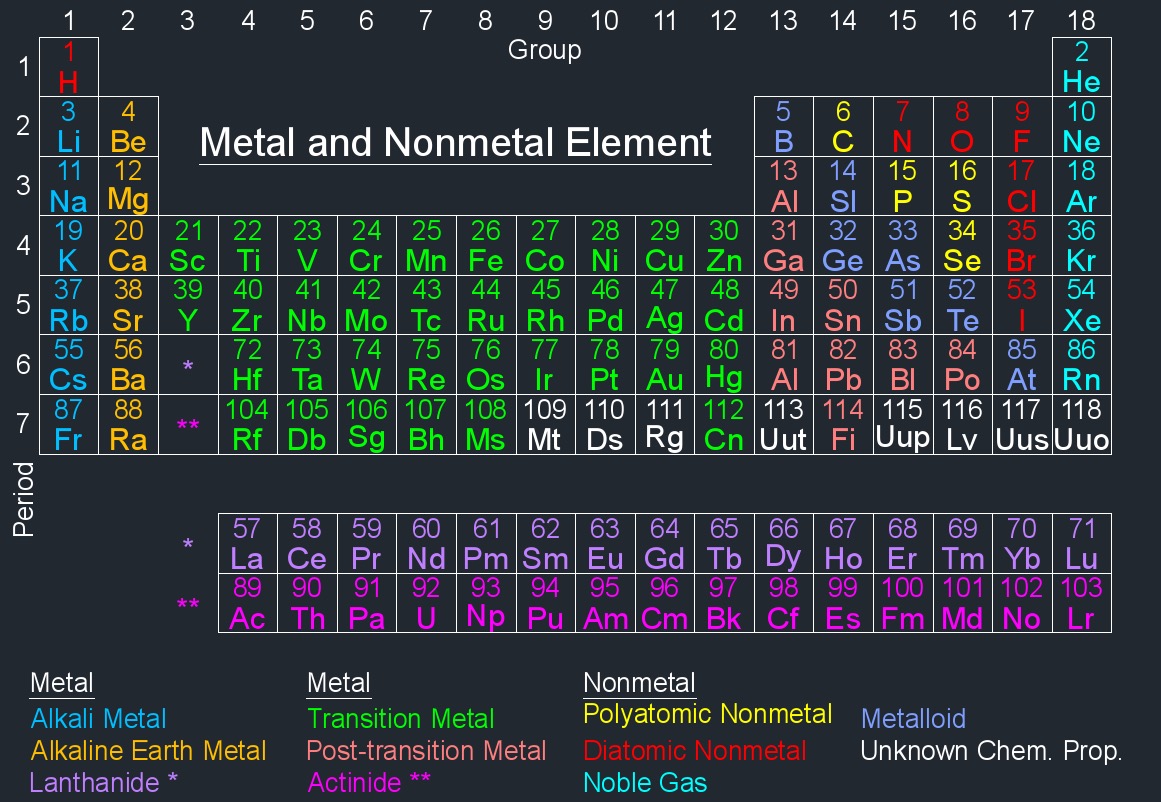 The physical properties of metal elements, abbreviated as \(MET\), have characteristic such as shiny, hard, high density, malleable, high melting point and can conduct electricity and heat well.
The physical properties of metal elements, abbreviated as \(MET\), have characteristic such as shiny, hard, high density, malleable, high melting point and can conduct electricity and heat well.
Actinide Element | ||||
|---|---|---|---|---|
| Element Name | Element Symbol | Atomic Number | Periodic Group Number | Periodic Period Number |
| Actinium | Ac | 89 | - | 7 |
| Americium | Am | 95 | - | 7 |
| Berkelium | Bk | 97 | - | 7 |
| Californium | Cf | 98 | - | 7 |
| Curium | Cm | 96 | - | 7 |
| Einsteinium | Es | 99 | - | 7 |
| Fermium | Fm | 100 | - | 7 |
| Mendelevium | Md | 101 | - | 7 |
| Neptunium | Np | 93 | - | 7 |
| Nobelium | No | 102 | - | 7 |
| Plutonium | Pu | 94 | - | 7 |
| Protactinium | Pa | 91 | - | 7 |
| Thorium | Th | 90 | - | 7 |
| Uranium | U | 92 | - | 7 |
Actinide
The actinide elements are a series of chemical elements found in Group 3 of the periodic table, which is in the f-block. This series includes elements with atomic numbers from 89 to 103. The actinides are often considered together because they share similar properties, particularly in terms of their electronic structure. These elements are typically characterized by the filling of the 5f orbitals.
Actinides have important applications, especially in the field of nuclear energy. Uranium, for example, is a key fuel in nuclear reactors. Plutonium, another actinide, is also used as a nuclear fuel and in the production of nuclear weapons. The study of actinide chemistry is significant in understanding the behavior of heavy radioactive elements and their compounds. Due to their radioactivity, handling and managing actinides present challenges, and there are environmental and safety concerns associated with their use.
Alkali Metal Element | ||||
|---|---|---|---|---|
| Element Name | Element Symbol | Atomic Number | Periodic Group Number | Periodic Period Number |
| Caesium | Cs | 55 | 1 | 6 |
| Francium | Fr | 87 | 1 | 7 |
| Lithium | Li | 3 | 1 | 2 |
| Potassium | K | 19 | 1 | 4 |
| Rubidium | Rb | 37 | 1 | 5 |
| Sodium | Na | 11 | 1 | 3 |
Alkali Metal
The alkali metals are found in Group 1 of the Periodic Table and they are highly reactive and usually found with other elements in nature, rarely by themselves. These are silvery colored, soft, low density metals. These elements are highly reactive metals, and they are so named because they form alkaline solutions (hydroxides) when they react with water. Hydrogen is also found in group 1, even though it is classified as a Other Nonmetal. It rarely has the characteristics of an alkali metal, but when it is under extremely high pressure it acts like one.
Key Points about Alkali Metals
Softness - Alkali metals are generally soft and can be easily cut with a knife. This is due to their relatively weak metallic bonding, which allows their atoms to slide past each other.
Low Density - Alkali metals have low densities compared to many other metals. For example, lithium is the least dense solid element.
Low Melting and Boiling Points - Alkali metals generally have low melting and boiling points compared to other metals. This makes them suitable for various applications, such as in heat transfer systems.
High Reactivity - Alkali metals are highly reactive, especially with water. When alkali metals react with water, they produce hydrogen gas and an alkaline solution. The reactivity increases down the group.
Single Valence Electron - All alkali metals have a single valence electron in their outermost electron shell, which makes them prone to losing that electron to form a positive ion.
These metals are crucial in various industrial applications. For example, sodium and potassium are essential for biological processes in living organisms, and they are commonly found in salts. Additionally, alkali metals like lithium are used in batteries, and cesium finds applications in atomic clocks. However, due to their high reactivity, alkali metals are usually stored and handled with care to prevent reactions with moisture and air.
Alkaline Earth Metal Element | ||||
|---|---|---|---|---|
| Element Name | Element Symbol | Atomic Number | Periodic Group Number | Periodic Period Number |
| Barium | Ba | 56 | 2 | 6 |
| Beryllium | Be | 4 | 2 | 2 |
| Calcium | Ca | 20 | 2 | 4 |
| Magnesium | Mg | 12 | 2 | 3 |
| Radium | Ra | 88 | 2 | 7 |
| Strontium | Sr | 38 | 2 | 5 |
The alkaline earth metals are a group of chemical elements found in Group 2 of the periodic table. These elements are silver colored, soft metals that will only melt at very high temperatures. These elements share certain similarities in their chemical properties due to their common electron configuration in the outermost electron shell.
Key Points about Alkaline Earth Metals
Reactivity - Alkaline earth metals are reactive but less so than the alkali metals in Group 1. They readily form compounds, especially oxides and hydroxides, with oxygen and water.
Single Valence Electron - Like the alkali metals, alkaline earth metals also have a relatively low number of electrons in their outermost electron shell. In this case, there are two valence electrons.
Increasing Atomic Size - The atomic size increases down the group. As you move from beryllium to radium, the atoms become larger.
Low Density: Alkaline earth metals generally have low densities, and they are lighter than most transition metals.
Higher Melting and Boiling Points - Compared to alkali metals, alkaline earth metals have higher melting and boiling points, reflecting the stronger metallic bonding in these elements.
Use in Alloys - Some alkaline earth metals, particularly magnesium and calcium, are used in alloys to enhance the properties of metals.
Biological Importance - Calcium and magnesium are essential elements for living organisms. Calcium is crucial for bone and teeth formation, blood clotting, and muscle function, while magnesium is a component of chlorophyll in plants and is important for various cellular processes.
Radium's Radioactivity - Radium, the heaviest element in this group, is radioactive. It was discovered by Marie Curie and Pierre Curie in 1898 and was once used in luminous paint due to its natural radioactivity. However, its use has declined due to its health risks.
Alkaline earth metals play important roles in various industrial, biological, and environmental processes. Understanding their properties and reactivity is essential for applications ranging from construction materials to medical treatments.
Lanthanide Element | ||||
|---|---|---|---|---|
| Element Name | Element Symbol | Atomic Number | Periodic Group Number | Periodic Period Number |
| Cerium | Ce | 58 | - | 6 |
| Dysprosium | Dy | 66 | - | 6 |
| Erbium | Er | 68 | - | 6 |
| Europium | Eu | 63 | - | 6 |
| Gadolinium | Gd | 64 | - | 6 |
| Holmium | Ho | 67 | - | 6 |
| Lanthanum | La | 57 | - | 6 |
| Neodymium | Nd | 60 | - | 6 |
| Praseodymium | Pr | 59 | - | 6 |
| Promethium | Pm | 61 | - | 6 |
| Samarium | Sm | 62 | - | 6 |
| Terbium | Tb | 65 | - | 6 |
| Thulium | Tm | 69 | - | 6 |
| Ytterbium | Yb | 70 | - | 6 |
Lanthanide
The lanthanides, also known as the lanthanoid series or rare earth elements, are a group of chemical elements found in the f-block of the periodic table.
Key Points about Lanthanide Elements
Similar Properties - The lanthanides share similar chemical properties due to the filling of their 4f electron orbitals. This results in comparable atomic and ionic sizes.
Metallic Nature - Lanthanides are metals with shiny and silvery appearances. They are generally good conductors of electricity.
High Magnetic Susceptibility: - Many lanthanides exhibit high magnetic susceptibility, making them useful in various applications, including the production of strong permanent magnets.
Use in Technology - Lanthanides find applications in various technologies. For example, neodymium and samarium are used in the production of powerful magnets, europium is used in phosphors for color television tubes, and gadolinium is used in magnetic resonance imaging (MRI) contrast agents.
Complex Chemistry - Lanthanides have complex coordination chemistry due to the presence of multiple oxidation states and the similarity of their electron configurations.
Promethium's Radioactivity - Promethium is the only lanthanide that does not have a stable isotope. It is radioactive and does not occur naturally in large quantities.
It's worth noting that the lanthanides are distinct from the actinides, which are another series of elements found in the f-block of the periodic table. Both lanthanides and actinides are important in various scientific, industrial, and technological applications, and they exhibit unique properties due to the filling of their respective f-orbitals
Post-Transition Metal Element | ||||
|---|---|---|---|---|
| Element Name | Element Symbol | Atomic Number | Periodic Group Number | Periodic Period Number |
| Aluminum | Al | 13 | 13 | 3 |
| Bismuth | Bi | 83 | 15 | 6 |
| Gallium | Ga | 31 | 13 | 4 |
| Indium | In | 49 | 13 | 5 |
| Lead | Pb | 82 | 14 | 6 |
| Thallium | Tl | 81 | 13 | 6 |
| Tin | Sn | 50 | 14 | 5 |
| Ununhexium | Uuh | 116 | 16 | 7 |
| Ununpentium | Uup | 115 | 15 | 7 |
| Ununquadium | Uuq | 114 | 14 | 7 |
| Ununtrium | Uut | 113 | 13 | 7 |
Post-transition Metal
Post-transition metals are elements found in the periodic table between the transition metals and the metalloids. They include elements from groups 13 to 16 (boron to radon).
Key Points about Post-transition Metals
Metallic Properties - Like transition metals, post-transition metals are generally metallic in nature. They have metallic luster, conduct electricity, and tend to form positive ions.
Lower Melting and Boiling Points - Compared to the transition metals, post-transition metals often have lower melting and boiling points.
Varied Properties - Post-transition metals exhibit a range of properties. For example, boron (B) and aluminum (Al) are metalloids or have some metalloid-like properties, while gallium (Ga) and indium (In) are true metals. Tin (Sn) and lead (Pb) are post-transition metals with distinctive properties.
Ductility and Malleability - Some post-transition metals, such as tin and lead, are ductile and malleable, meaning they can be stretched into thin wires or shaped into thin sheets.
Metallic Bonding - Post-transition metals typically form metallic bonds due to the delocalization of electrons within the metal lattice.
These elements often display a mix of metallic and non-metallic properties, and they are important in various industrial applications. For instance, aluminum is widely used in the aerospace and construction industries, tin is used in soldering, and lead has been historically used in a variety of applications, although its use is now limited due to environmental concerns.
Transition Metal Element | ||||
|---|---|---|---|---|
| Element Name | Element Symbol | Atomic Number | Periodic Group Number | Periodic Period Number |
| Bohrium | Bh | 107 | 7 | 7 |
| Cadmium | Cd | 48 | 12 | 5 |
| Chromium | Cr | 24 | 6 | 4 |
| Cobalt | Co | 27 | 9 | 4 |
| Copper | Cu | 29 | 11 | 4 |
| Dubnium | Db | 105 | 5 | 7 |
| Gold | Au | 79 | 11 | 6 |
| Hahnium | Hf | 72 | 4 | 6 |
| Hassium | Hs | 108 | 8 | 7 |
| Iridium | Ir | 77 | 9 | 6 |
| Iron | Fe | 26 | 8 | 4 |
| Manganese | Mn | 25 | 7 | 4 |
| Mercury | Hg | 80 | 12 | 6 |
| Molybdenum | Mo | 42 | 6 | 5 |
| Nickel | Ni | 28 | 10 | 4 |
| Niobium | Nb | 41 | 5 | 5 |
| Osmium | Os | 76 | 8 | 6 |
| Palladium | Pd | 46 | 10 | 5 |
| Platinum | Pt | 78 | 10 | 6 |
| Rhenium | Re | 75 | 7 | 6 |
| Rhodium | Rh | 45 | 9 | 5 |
| Rutherfordium | Rf | 104 | 4 | 7 |
| Ruthenium | Ru | 44 | 8 | 5 |
| Scandium | Sc | 21 | 3 | 4 |
| Seaborgium | Sg | 106 | 6 | 7 |
| Silver | Ag | 47 | 11 | 5 |
| Tantalum | Ta | 73 | 5 | 6 |
| Technetium | Tc | 43 | 7 | 5 |
| Titanium | Ti | 22 | 4 | 4 |
| Tungsten | W | 74 | 6 | 6 |
| Ununbium | Uub | 112 | 12 | 7 |
| Vanadium | V | 23 | 5 | 4 |
| Yttrium | Y | 39 | 3 | 5 |
| Zinc | Zn | 30 | 12 | 4 |
| Zirconium | Zr | 40 | 4 | 5 |
Transition Metal
Transition metals are a group of elements found in the central portion of the periodic table, spanning from groups 3 to 12. These elements are known for their unique electronic configurations, particularly the filling of their d orbitals.
Key Points about Transition Metals
Variable Oxidation States - Transition metals can exhibit multiple oxidation states or valence states. The presence of partially filled d orbitals allows them to readily lose or gain electrons.
Formation of Colored Compounds - Transition metal compounds often display vibrant colors due to the transitions of electrons between d orbitals.
Complex Formation - Transition metals have a tendency to form complex ions and compounds. They can coordinate with ligands, forming coordination complexes with characteristic structures.
Paramagnetism - Many transition metals are paramagnetic, meaning they are attracted to a magnetic field due to the presence of unpaired electrons.
High Melting and Boiling Points - Transition metals generally have high melting and boiling points, contributing to their utility in various industrial applications.
Metallic Properties - Transition metals are good conductors of electricity and have metallic luster. They typically have high densities and are malleable and ductile.
Catalytic Activity - Many transition metals and their compounds serve as catalysts in chemical reactions. Their ability to change oxidation states makes them effective in facilitating reaction pathways.
Transition metals play crucial roles in various industrial processes, from catalysis to the production of alloys and electronic devices. They are found in everyday items, such as jewelry, coins, and electronics.

