Magnetic Element
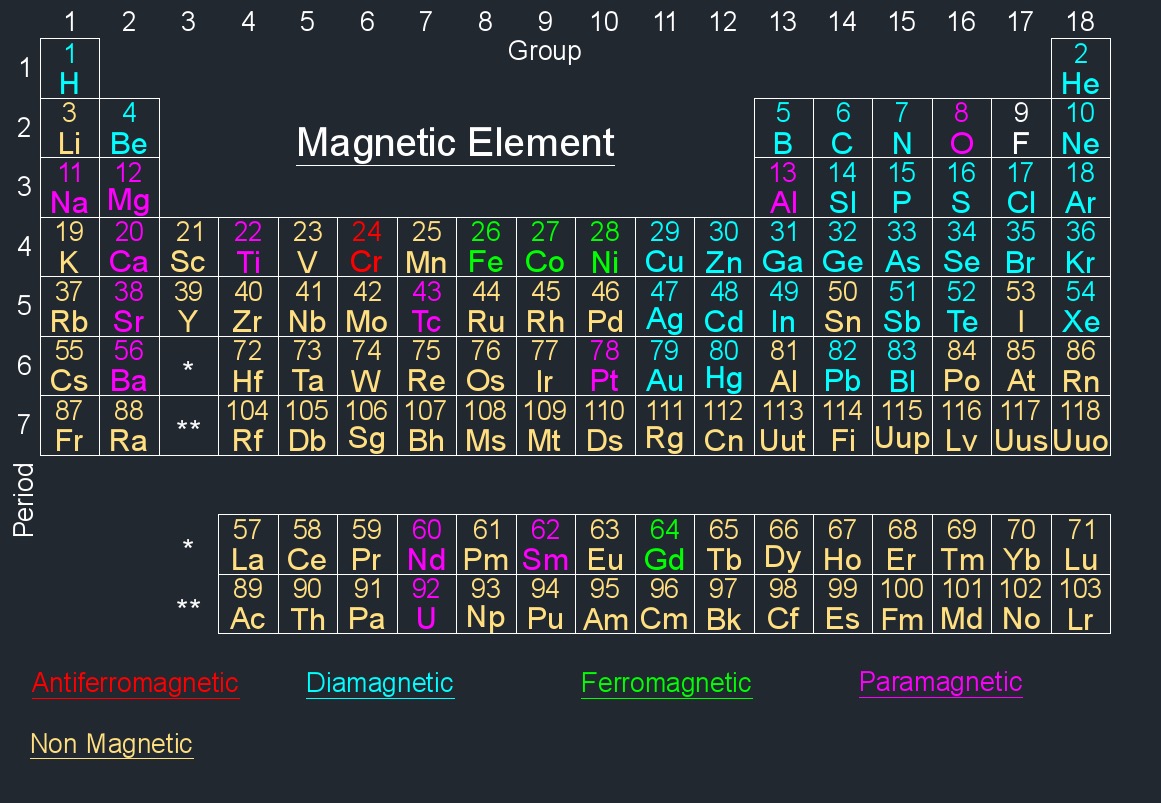 Magnetic elements exhibits magnetic properties. These properties arise from the presence of magnetic moments associated with the electrons in the atoms of the element. Magnetic moments result from the intrinsic spin of electrons and their orbital motion around the atomic nucleus.
Magnetic elements exhibits magnetic properties. These properties arise from the presence of magnetic moments associated with the electrons in the atoms of the element. Magnetic moments result from the intrinsic spin of electrons and their orbital motion around the atomic nucleus.
It's important to note that not all elements exhibit significant magnetic properties. Elements like oxygen and nitrogen, for example, are generally not magnetic under normal conditions.
The magnetic behavior of an element is influenced by factors such as electron configuration, spin, and the arrangement of electrons in the material's atomic structure. These properties have implications for various applications, including the development of magnetic materials for technology and industry.
Antiferromagnetic Element | ||||
|---|---|---|---|---|
| Element Name | Element Symbol | Atomic Number | Periodic Group Number | Periodic Period Number |
| Chromium | Cr | 24 | 6 | 4 |
Antiferromagnetism is a type of magnetic ordering in which neighboring magnetic moments in a material align in opposite directions. This means that the magnetic moments of adjacent atoms or ions within the material are oriented antiparallel to each other. As a result, the overall magnetic effect of an antiferromagnetic material is often canceled out.
In antiferromagnetic materials, adjacent magnetic moments tend to align in opposite directions, leading to a cancellation of the overall magnetic moment. This behavior is in contrast to ferromagnetic materials, where neighboring magnetic moments align parallel to each other, reinforcing the overall magnetization of the material. Antiferromagnetic materials are interesting from a scientific standpoint, and they have applications in various technological fields, including magnetic data storage and spintronics.
Diamagnetic Element | ||||
|---|---|---|---|---|
| Element Name | Element Symbol | Atomic Number | Periodic Group Number | Periodic Period Number |
| Antimony | Sb | 51 | 15 | 5 |
| Argon | Ar | 18 | 18 | 3 |
| Arsenic | As | 33 | 15 | 4 |
| Beryllium | Be | 4 | 2 | 2 |
| Bismuth | Bi | 83 | 15 | 6 |
| Boron | B | 5 | 13 | 2 |
| Bromine | Br | 35 | 17 | 4 |
| Carbon | C | 6 | 14 | 2 |
| Cadmium | Cd | 48 | 12 | 5 |
| Chlorine | Cl | 17 | 17 | 3 |
| Copper | Cu | 29 | 11 | 4 |
| Gallium | Ga | 31 | 13 | 4 |
| Germanium | Ge | 32 | 14 | 4 |
| Gold | Au | 79 | 11 | 6 |
| Helium | He | 2 | 18 | 1 |
| Hydrogen | H | 1 | 1 | 1 |
| Indium | In | 49 | 13 | 5 |
| Krypton | Kr | 36 | 18 | 4 |
| Lead | Pb | 82 | 14 | 6 |
| Neon | Ne | 10 | 18 | 2 |
| Mercury | Hg | 80 | 12 | 6 |
| Nitrogen | N | 7 | 15 | 2 |
| Phosphorus | P | 15 | 15 | 3 |
| Selenium | Se | 34 | 16 | 4 |
| Silicon | Si | 14 | 14 | 3 |
| Silver | Ag | 47 | 11 | 5 |
| Sulfur | S | 16 | 16 | 3 |
| Tellurium | Te | 52 | 16 | 5 |
| Xenon | Xe | 54 | 18 | 5 |
| Zinc | Zn | 30 | 12 | 4 |
Diamagnetism is a property of some materials that causes them to be repelled by a magnetic field. In diamagnetic substances, the magnetic moments of individual atoms or molecules are oriented in such a way that they oppose the external magnetic field. As a result, diamagnetic materials are weakly repelled by magnets.
Most elements and compounds exhibit some degree of diamagnetism, but it is often a very weak effect compared to other types of magnetism. For example, noble gases such as helium and neon are typically diamagnetic. When placed in a magnetic field, these elements show a very weak tendency to be repelled. Diamagnetic behavior arises due to the orbital motion of electrons within atoms or molecules. When an external magnetic field is applied, the electrons respond in a way that creates a weak opposing magnetic field, resulting in the observed repulsion. It's important to note that diamagnetic effects are generally weaker than paramagnetic or ferromagnetic effects.
Ferromagnetic Element | ||||
|---|---|---|---|---|
| Element Name | Element Symbol | Atomic Number | Periodic Group Number | Periodic Period Number |
| Cobalt | Co | 27 | 9 | 4 |
| Gadolinium | Gd | 64 | - | 6 |
| Iron | Fe | 26 | 8 | 4 |
| Nickel | Ni | 28 | 10 | 4 |
Ferromagnetism is a type of magnetic ordering in which adjacent magnetic moments in a material align parallel to each other. This alignment results in a strong and permanent magnetization of the material. Ferromagnetic materials can retain their magnetization even after the external magnetic field is removed. This property is what makes them useful in various applications, including the production of permanent magnets.
Ferromagnetic materials are crucial in various applications, including the production of permanent magnets used in electric motors, generators, and a wide range of electronic devices. Understanding the magnetic properties of ferromagnetic elements is fundamental to the development of magnetic materials for technological applications.
Paramagnetic Element | ||||
|---|---|---|---|---|
| Element Name | Element Symbol | Atomic Number | Periodic Group Number | Periodic Period Number |
| Aluminum | Al | 13 | 13 | 3 |
| Barium | Ba | 56 | 2 | 6 |
| Calcium | Ca | 20 | 2 | 4 |
| Magnesium | Mg | 12 | 2 | 3 |
| Neodymium | Nd | 60 | - | 6 |
| Oxygen | O | 8 | 16 | 2 |
| Platinum | Pt | 78 | 10 | 6 |
| Samarium | Sm | 62 | - | 6 |
| Sodium | Na | 11 | 1 | 3 |
| Strontium | Sr | 38 | 2 | 5 |
| Technetium | Tc | 43 | 7 | 5 |
| Titanium | Ti | 22 | 4 | 4 |
| Uranium | U | 92 | - | 7 |
Paramagnetism is a property of certain materials where the individual atomic or molecular magnetic dipoles align themselves with an external magnetic field. Unlike ferromagnetic materials, where neighboring magnetic moments align parallel to each other, paramagnetic materials exhibit a weak attraction to an external magnetic field. In paramagnetic substances, the magnetic moments associated with individual atoms or molecules tend to align in the direction of the applied magnetic field, but the alignment is relatively weak. When the external magnetic field is removed, the material loses its magnetic properties.
Paramagnetism arises due to the presence of unpaired electrons in the atomic or molecular orbitals. These unpaired electrons have magnetic moments that tend to align with an external magnetic field, resulting in a weak attraction.

