Unknown Chemical Element
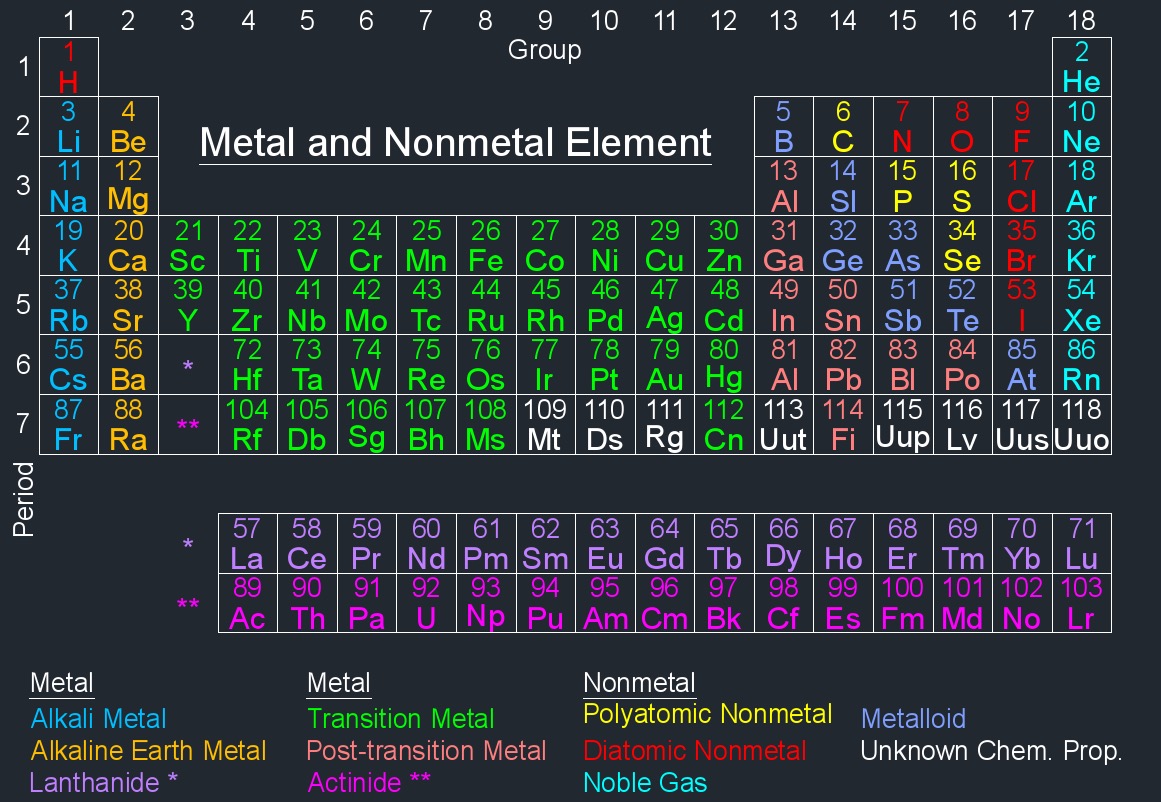
Unknown chemical elements are elements that have not yet been discovered, confirmed, or characterized. In the periodic table, all known elements have been identified and their properties studied. However, there is the possibility that new elements could be discovered, especially at the extreme ends of the periodic table, particularly in the area of superheavy elements (elements with very high atomic numbers).
Hypothetical Existence - Scientists may predict the existence of these elements based on trends in the periodic table or through theoretical models, even if they haven't been observed directly.
Superheavy Elements - Many unknown elements are likely to be superheavy, with atomic numbers greater than those currently known (beyond element 118, Oganesson). These elements might exist for extremely short periods due to instability.
Synthesis in Laboratories - Some unknown elements might be synthesized in laboratories through nuclear reactions. However, because they are highly unstable, they may decay into known elements almost immediately after their creation, making them difficult to study.
Challenges in Discovery - Detecting new elements requires advanced technology and techniques, such as particle accelerators, as these elements are often highly unstable and exist only for fractions of a second.
Naming - Once an unknown element is discovered and confirmed, it is typically given a temporary systematic name based on its atomic number until a permanent name is decided by the International Union of Pure and Applied Chemistry (IUPAC).
Unknown Chemical Element |
||||
| Element Name | Element Symbol | Atomic Number | Periodic Group Number | Periodic Period Number |
| Darmstadtium | Ds | 110 | 10 | 7 |
| Meitnerium | Mt | 109 | 9 | 7 |
| Roentgenium | Rg | 111 | 11 | 7 |
| Ununhexium | Uuh | 116 | 16 | 7 |
| Ununoctium | Uuo | 118 | 18 | 7 |
| Ununpentium | Uup | 115 | 15 | 7 |
| Ununseptium | Uus | 117 | 17 | 7 |
| Ununtrium | Uut | 113 | 13 | 7 |

