Quantum Mechanics
Physics, Glossary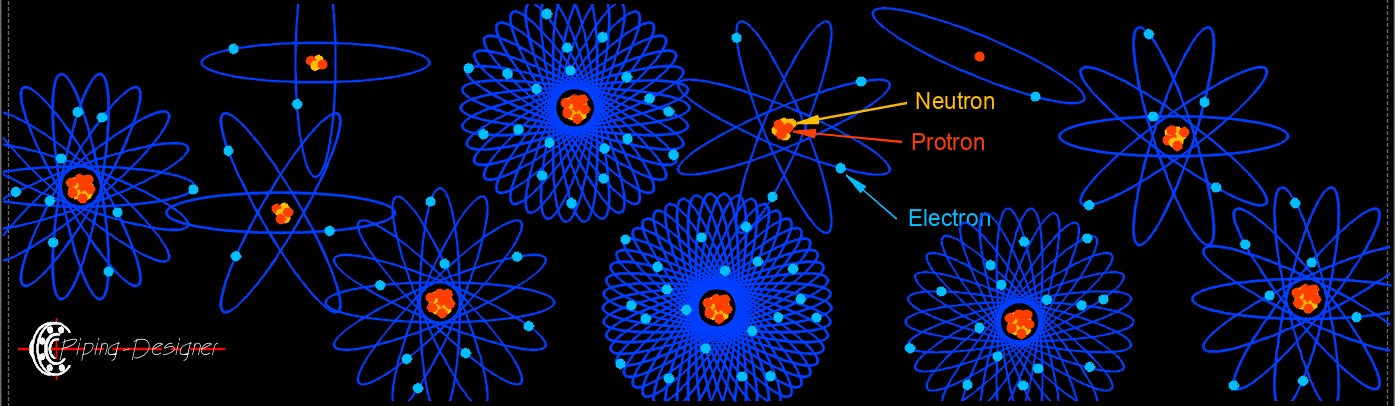 Quantum mechanics is the branch of physics that describes the behavior of matter and energy at the atomic and subatomic level. It is a fundamental theory that provides a framework for understanding the behavior of particles such as electrons, protons, and photons, and their interactions with each other. It is based on the principle of wave particle duality, which states that particles can exhibit both wave like and particle like behavior depending on how they are observed. It also introduces the concept of probability into the description of physical systems, with the behavior of particles being described in terms of probability amplitudes rather than definite positions or trajectories.
Quantum mechanics is the branch of physics that describes the behavior of matter and energy at the atomic and subatomic level. It is a fundamental theory that provides a framework for understanding the behavior of particles such as electrons, protons, and photons, and their interactions with each other. It is based on the principle of wave particle duality, which states that particles can exhibit both wave like and particle like behavior depending on how they are observed. It also introduces the concept of probability into the description of physical systems, with the behavior of particles being described in terms of probability amplitudes rather than definite positions or trajectories.
| Physics |
The mathematics of quantum mechanics is based on wave functions, which describe the probability amplitude of a particle being in a particular state or location. These wave functions can be used to predict the behavior of particles and the outcomes of experiments with a high degree of accuracy. Quantum mechanics has numerous practical applications, including the development of semiconductor devices, lasers, and medical imaging technologies. It is also fundamental to our understanding of many phenomena in physics, including the behavior of atoms and molecules, the structure of matter, and the nature of light.
Atomic Properties
- Atoms (see periodic table) are made up of electrons, neutrons, and protons called subatomic particles.
- Atoms can be joined togeather to create molecules.
- Atoms have both nutrons and protons in the necleus.
- A neutral atom or normal atom has an equal number of electrons (-) and nuetrons (+).
- An ion has an unequal number of electrons and nuetrons (more or less electrons). Positive charge if more protons than electrons (missing electrons). Negative charge if less protons than electrons (extra electrons).
- Atoms have an equal number of protons and electrons since the electrical charge has to balance out.
- Atomic weight is the total number of particles in an atom's nucleus or specifically the average weight of naturally occuring isatobes of an element related to the mass.
- The atomic number or proton number is the number of protons in an atom of the element.
- All atoms have neutrons except for hydrogen atoms.
- Electrons have a negative electrical charge.
- Electrons orbit the neucleus of an atom.
- Electrons are 1,800 times smaller than neutrons and protons.
- Neutrons have no charge.
- To find the number of nuetrons if you know the isatobe of the atom, subtract the number of protons from the mass number.
- Isatobes are created when you change the normal number of nuetrons in an atom.
- Protrons have a positive electrical charge.
- One proton is about 1,835 times more massive than an electron.
- Protrons and newtrons normally stick togeather except in radioactive decay when thay may be knocked out.

