Henry's Law
Henry's Law Formula |
||
|
\( C \;=\; k \cdot P \) (Henry's Law) \( k \;=\; \dfrac{ C }{ P }\) \( P \;=\; \dfrac{ C }{ k }\) |
||
| Symbol | English | Metric |
| \( C \) = Concentration of Disolverd Gas | - | \(Molarity\) |
| \( k \) = Henry's Law Constant | - | \(M\;/\;atm\) |
| \( P \) = Partial Pressure of the Gas above the Liquid | - | \(atm\) |
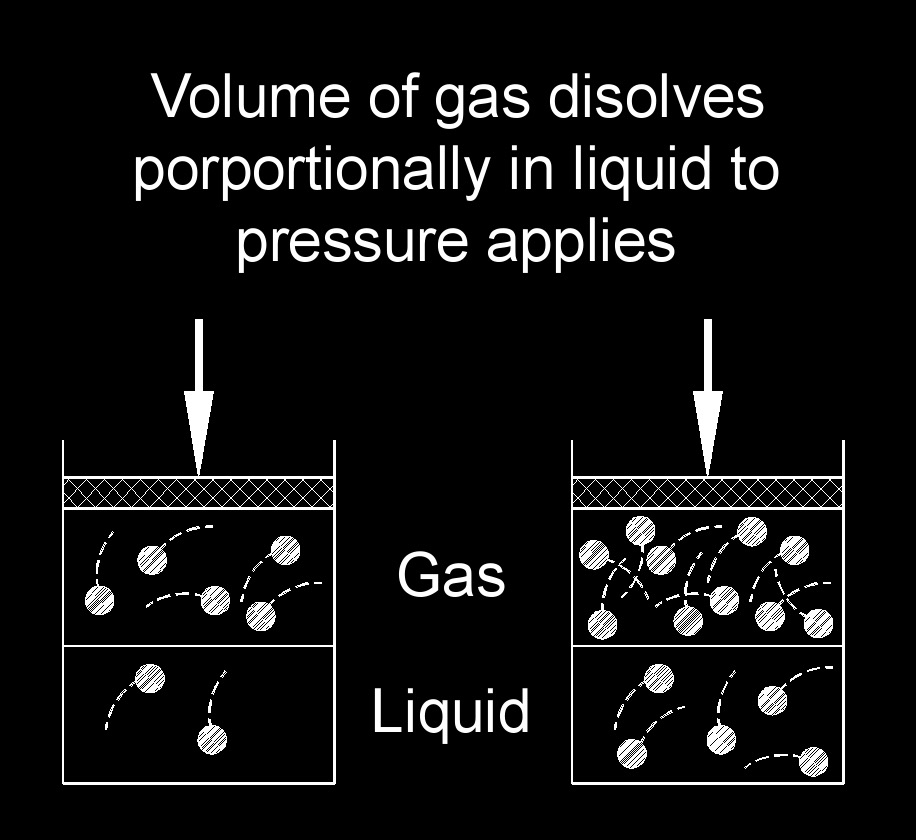 Henry's law, also called law of gas solubillity, describes the relationship between the concentration of a gas dissolved in a liquid and the partial pressure of that gas above the liquid. It states that the concentration of a gas in a solution is directly proportional to the partial pressure of the gas, provided that the temperature remains constant. In simpler terms, Henry's law states that the higher the partial pressure of a gas, the more of that gas will dissolve in a liquid. This relationship is particularly applicable to gases that are sparingly soluble in liquids, such as oxygen and carbon dioxide in water.
Henry's law, also called law of gas solubillity, describes the relationship between the concentration of a gas dissolved in a liquid and the partial pressure of that gas above the liquid. It states that the concentration of a gas in a solution is directly proportional to the partial pressure of the gas, provided that the temperature remains constant. In simpler terms, Henry's law states that the higher the partial pressure of a gas, the more of that gas will dissolve in a liquid. This relationship is particularly applicable to gases that are sparingly soluble in liquids, such as oxygen and carbon dioxide in water.
Henry's law has numerous applications, including in the field of environmental science, where it helps explain the dissolution of gases in bodies of water, as well as in various industrial processes and technologies, such as gas absorption and purification techniques.

