Gas
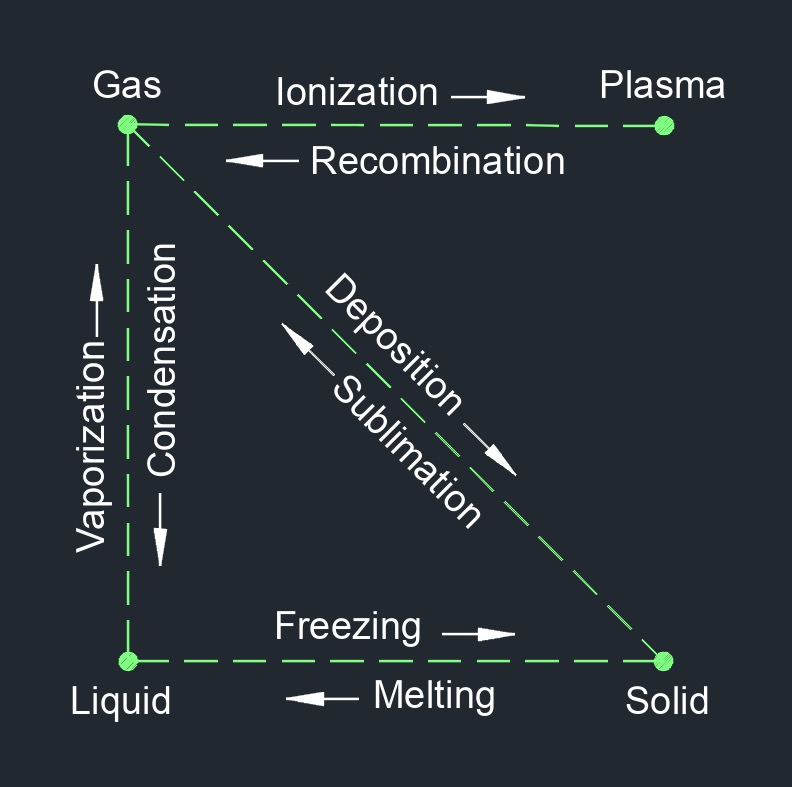
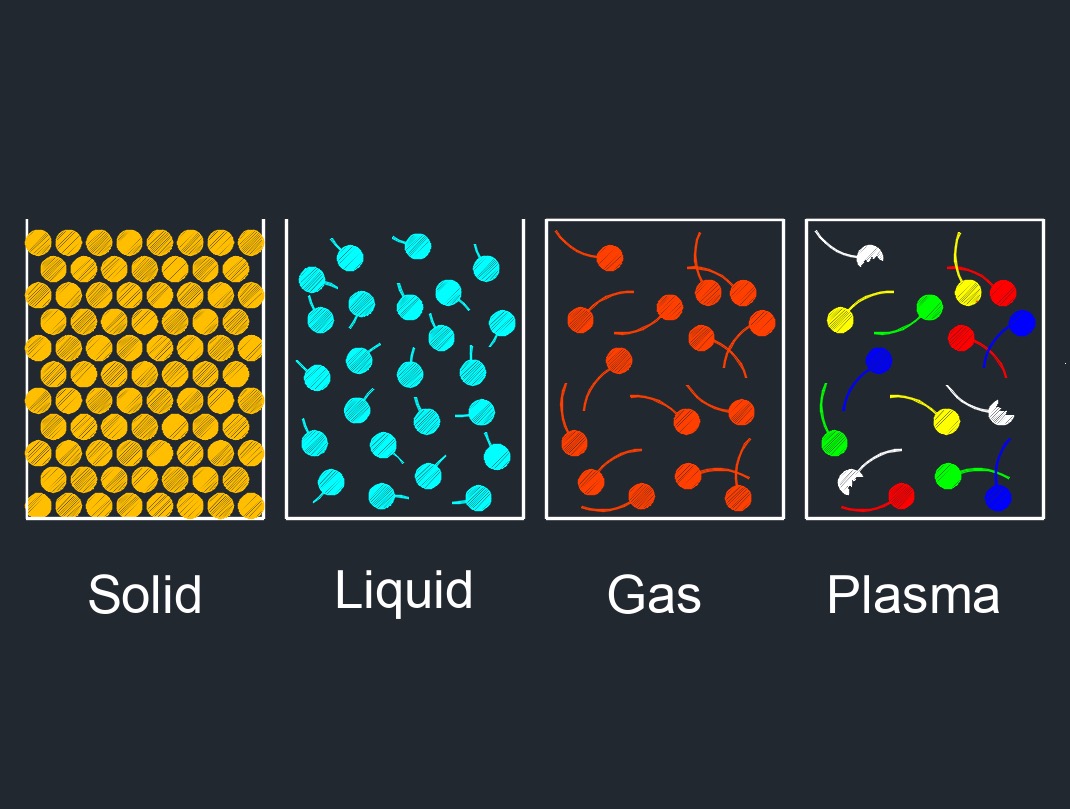 Gas, abbreviated as \(G\), is able to be compressed to fit a confined space and expanded when released. Gas is one of the three common states of matter, along with solid and liquid.
Gas, abbreviated as \(G\), is able to be compressed to fit a confined space and expanded when released. Gas is one of the three common states of matter, along with solid and liquid.
In its gaseous state, matter has no fixed shape or volume, and it fills its container completely, taking the shape of the container. Gases are compressible and have low density compared to solids and liquids. Gases are made up of molecules or atoms that are in constant motion and collide with each other and with the walls of their container. The behavior of a gas can be described by a few key properties, such as pressure, volume, temperature, and the number of molecules or atoms present.
Gas Properties |
|||
| Properties | Gas | Liquid | Solid |
| Compressibility | Yes | Low | No |
| Density | Low | Moderate | High |
| Expandability | Yes | Yes | No |
| Intermolecule force strength | Weak | Moderate | Strong |
| Particle Movement | Free movement | Free movement | No free movement |
| Shape | Infinite | Infinite | Fixed |
| Shear Resistance | Yes | Yes | No |
| Viscosity | Low | Varies | No |
| Volume | Infinite | Fixed | Fixed |
Gas is an important substance in many areas of science and technology, including chemistry, physics, engineering, and environmental science. It is used as a fuel for heating, cooking, and transportation, and as a raw material for many industrial processes. The behavior of gases is also important in understanding the behavior of the atmosphere and the earth's climate.

