Sublimation
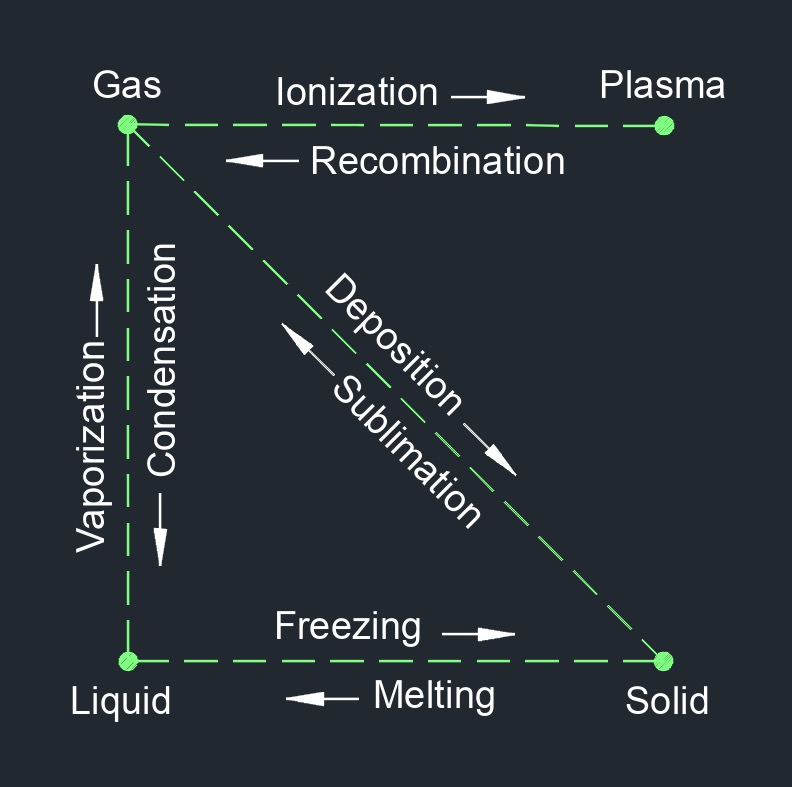
 Sublimation is a physical change of a substance from a solid phase to a gas phase and does not pass through the transitional liquid phase. This is an endothermic phase transition that occures at temperatures and pressures below a substance's triple point in its phase diagram. Since this transition goes straight from solid to gas it has to happen immediately. Triple point of a substance is when the temperature and the pressure of all three phases, gas, liquid, and solid, coexist in thermodynamic equilibrium.
Sublimation is a physical change of a substance from a solid phase to a gas phase and does not pass through the transitional liquid phase. This is an endothermic phase transition that occures at temperatures and pressures below a substance's triple point in its phase diagram. Since this transition goes straight from solid to gas it has to happen immediately. Triple point of a substance is when the temperature and the pressure of all three phases, gas, liquid, and solid, coexist in thermodynamic equilibrium.
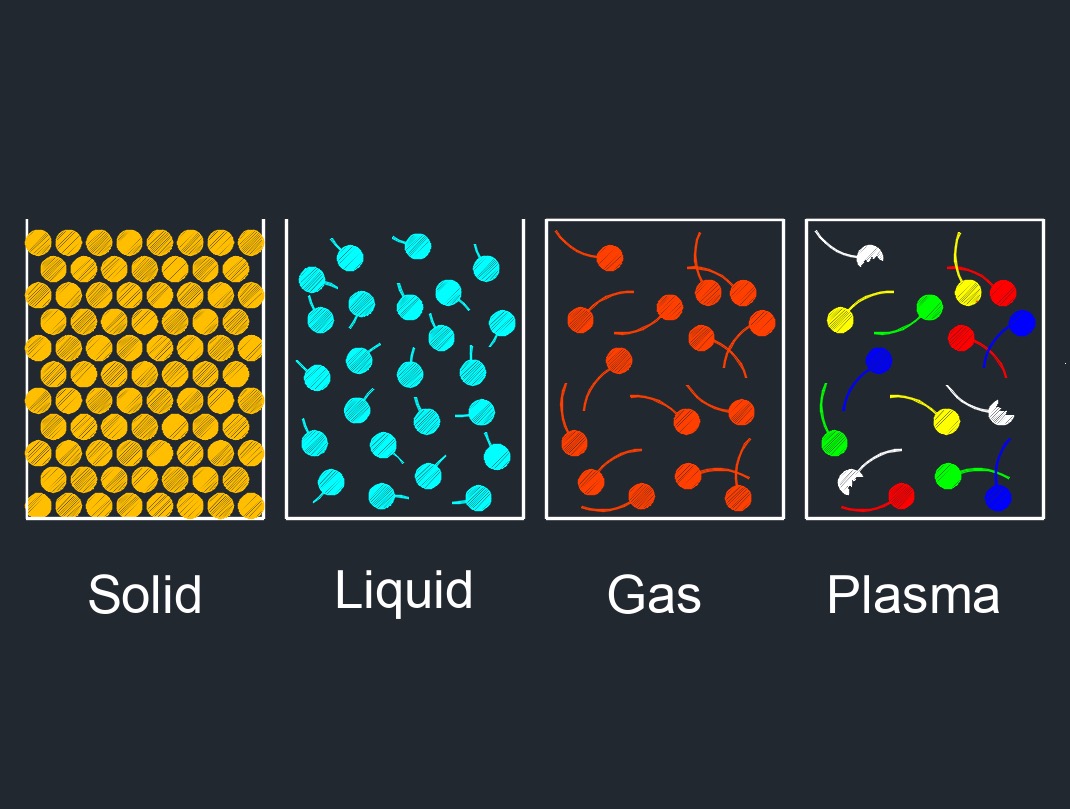
There are four phases of matter: gas, liquid, plasma, and solid.

