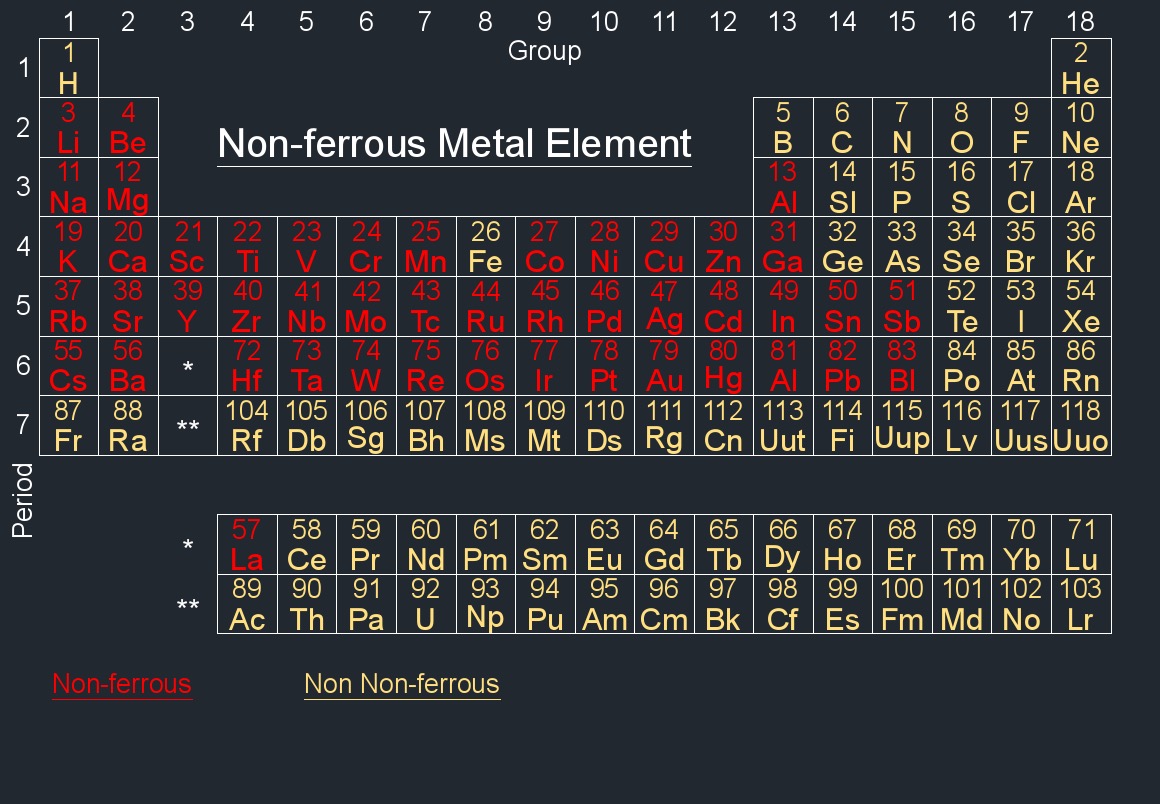
Periodic Table
The periodic table is a tabular arrangement of chemical elements, organized based on their atomic number, electron configuration, and recurring chemical properties. The table allows scientists and students to understand the relationships between various elements, providing a systematic way to study and predict the behavior of elements in chemical reactions.
Key Features of the Periodic Table
Rows (Periods) - Horizontal rows in the periodic table are called periods. Each period represents a new energy level or shell of electrons.
Columns (Groups) - Vertical columns in the periodic table are called groups. Elements within the same group share similar chemical properties because they have the same number of valence electrons.
Blocks - The periodic table is divided into blocks based on the electron subshells being filled. The main blocks are s, p, d, and f.
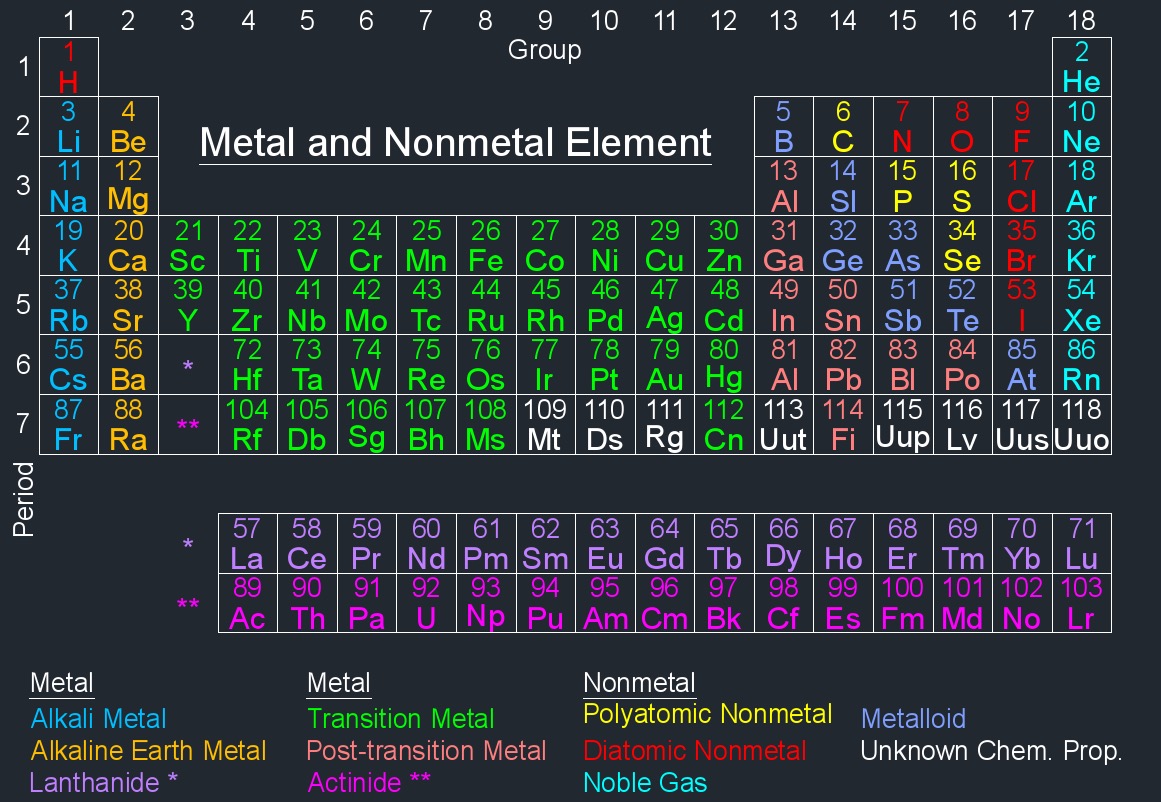
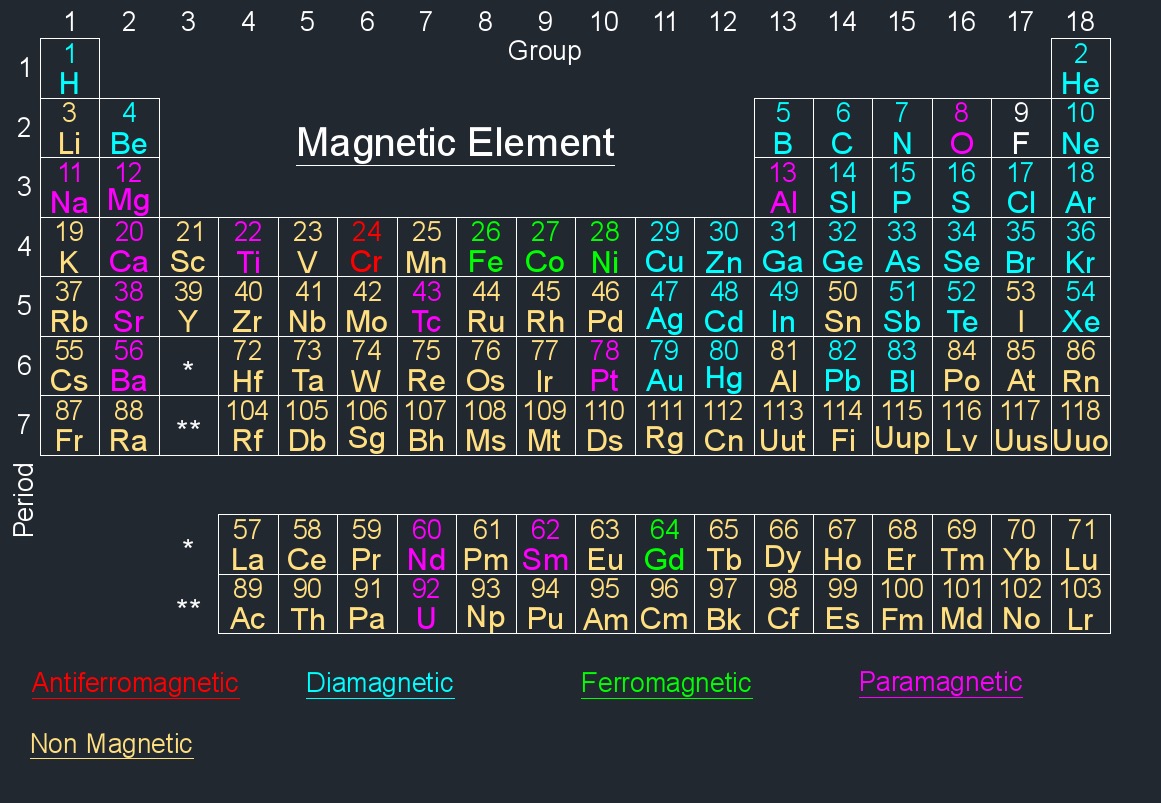
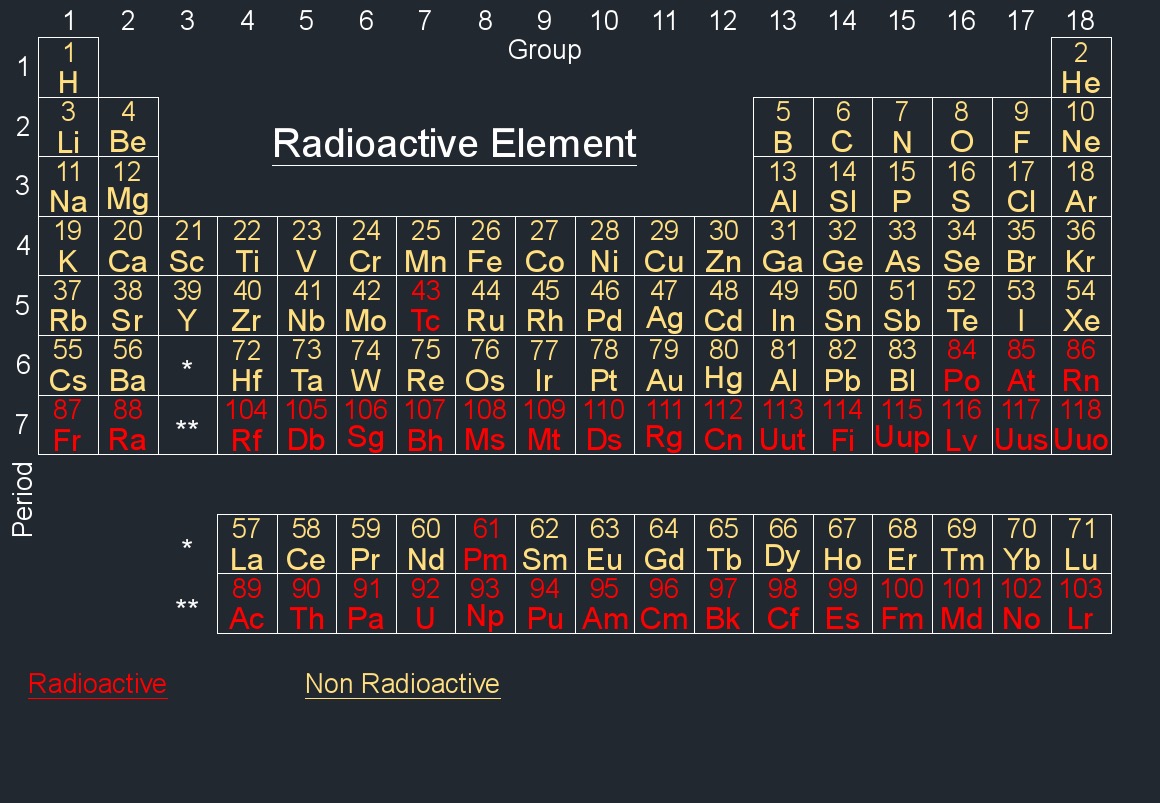
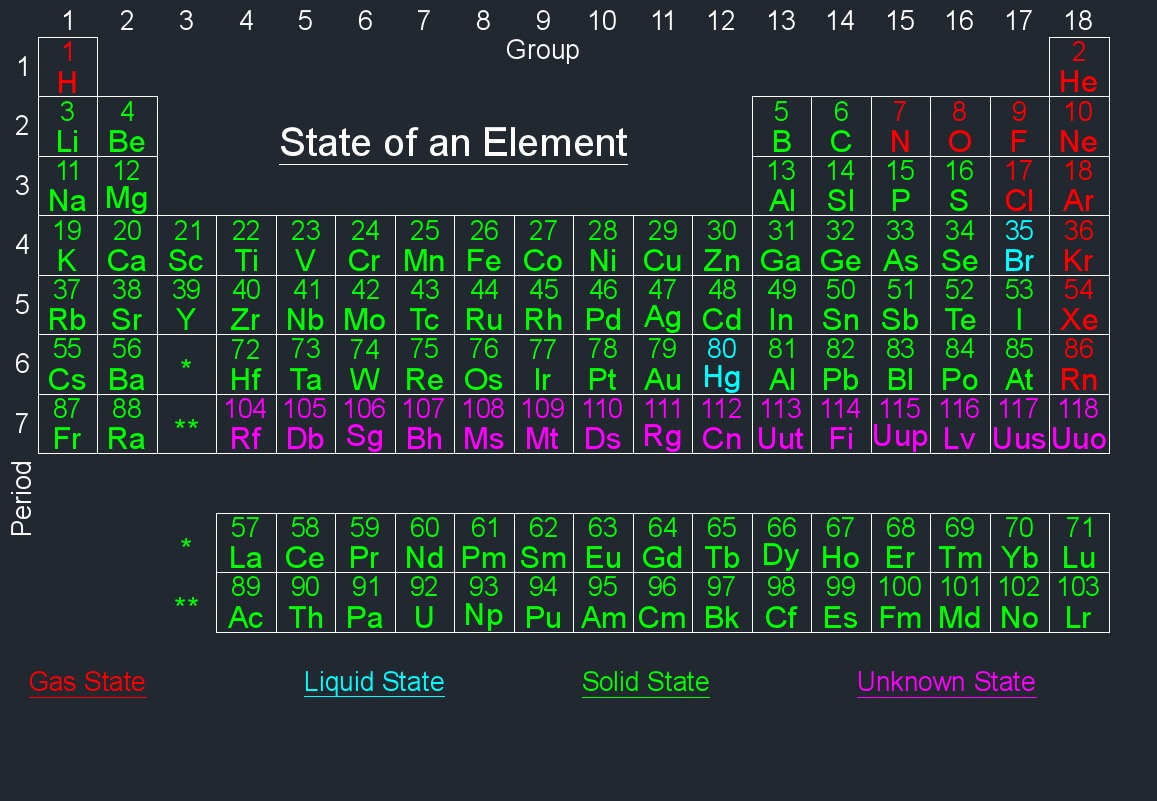
Element Information - Each element is represented by a box containing its atomic number, symbol, and atomic mass. The atomic number represents the number of protons in an atom's nucleus, and the atomic mass is the weighted average mass of an element's isotopes.
The modern periodic table is arranged based on the electronic configuration of atoms, which reflects their outermost electron shell and chemical behavior. Elements are ordered by increasing atomic number (the number of protons in an atom).
Dmitri Mendeleev is credited with the early development of the periodic table in the late 19th century. The modern version has been refined and expanded, providing a comprehensive framework for understanding the relationships between elements and facilitating the prediction of the properties of undiscovered elements.