Initial Temperature
Initial Temperature Formula |
||
|
\( T_i \;=\; T_f - \Delta T \) (Initial Temperature) \( T_f \;=\; T_i + \Delta T \) \( \Delta T \;=\; T_f - T_i \) |
||
| Symbol | English | Metric |
| \( T_i \) = Initial Temperature | \(^\circ F\) | \(^\circ C\) |
| \( T_f \) = Final Temperature | \(^\circ F\) | \(^\circ C\) |
| \( \Delta T \) = Change in Temperature | \(^\circ F\) | \(^\circ C\) |
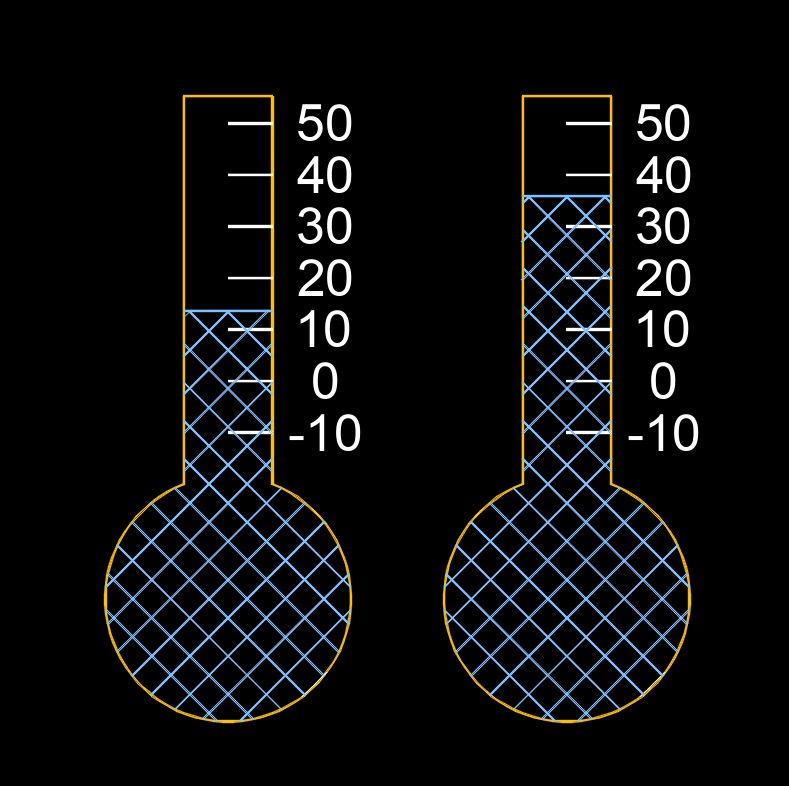
Initial Temperature Formula |
||
|
\( T_i \;=\; T_f - \dfrac{ Q }{ c \cdot m} \) (Initial Temperature) \( T_f \;=\; T_i + \dfrac{ Q }{ c \cdot m} \) \( Q \;=\; c \cdot m \cdot ( T_f - T_i ) \) \( c \;=\; \dfrac{ Q }{ m \cdot ( T_f - T_i ) } \) \( m \;=\; \dfrac{ Q }{ c \cdot ( T_f - T_i ) } \) |
||
| Symbol | English | Metric |
| \( T_i \) = Initial Temperature | \(^\circ F\) | \(^\circ C\) |
| \( T_f \) = Final Temperature | \(^\circ F\) | \(^\circ C\) |
| \( Q \) = Specific Heat Capacity | \(Btu \;/\; lbm-F\) | \(kJ \;/\;kg-K\) |
| \( c \) = Specific Heat | \(Btu \;/\; lbm-F\) | \(kJ \;/\;kg-K\) |
| \( m \) = Object Mass | \(lbm\) | \(kg\) |
Initial temperature, abbreviated as \(T_i\) or \(T_{intial}\), is the starting temperature of a substance, object, or system before any changes occur. It is the temperature measured at the beginning of a process, experiment, or event, and it serves as the reference point for comparing how much the temperature increases or decreases. Knowing the initial temperature is important because it allows scientists and engineers to determine the change in temperature, calculate heat transfer, predict material behavior, and analyze thermal processes. It essentially anchors all temperature related comparisons by establishing where the system began before any heating, cooling, or transformation took place.

Initial Temperature Formula |
||
|
\( T_i \;=\; T_f - \dfrac{ \Delta L }{ \overrightarrow{\alpha_l} \cdot L_o } \) (Initial Temperature) \( T_f \;=\; T_i + \dfrac{ \Delta L }{ \overrightarrow{\alpha_l} \cdot L_o } \) \( \Delta L \;=\; \overrightarrow{\alpha_l} \cdot L_o \cdot ( T_f - T_i ) \) \( \overrightarrow{\alpha_l} \;=\; \dfrac{ \Delta L }{ L_o \cdot ( T_f - T_i ) } \) \( L_o \;=\; \dfrac{ \Delta L }{ \overrightarrow{\alpha_l} \cdot ( T_f - T_i ) } \) |
||
| Symbol | English | Metric |
| \( T_i \) = Initial Temperature | \(^\circ F\) | \(^\circ C\) |
| \( T_f \) = Final Temperature | \(^\circ F\) | \(^\circ C\) |
| \( \Delta L \) = Change in Length | \(ft\) | \(m\) |
| \( \overrightarrow{\alpha_l} \) (Greek symbol alpha) = Linear Thermal Expansion Coefficient | \(in \;/\; in\;F\) | \(mm \;/\; mm\;C\) |
| \( L_o \) = Origional Length | \(ft\) | \(m\) |
Initial Temperature Formula |
||
|
\( T_i \;=\; T_f - \dfrac{ \Delta V }{ \beta_v \cdot V_o } \) (Initial Temperature) \( T_f \;=\; T_i + \dfrac{ \Delta V }{ \beta_v \cdot V_o } \) \( \Delta V \;=\; \beta_v \cdot V_o \cdot ( T_f - T_i ) \) \( \beta_v \;=\; \dfrac{ \Delta V }{ V_o \cdot ( T_f - T_i ) } \) \( V_o \;=\; \dfrac{ \Delta V }{ \beta_v \cdot ( T_f - T_i ) } \) |
||
| Symbol | English | Metric |
| \( T_i \) = Initial Temperature | \(^\circ F\) | \(^\circ C\) |
| \( T_f \) = Final Temperature | \(^\circ F\) | \(^\circ C\) |
| \( \Delta V \) = Change in Volume | \(ft^3\) | \(m^3\) |
| \( \beta_v \) = Volumetric Thermal Expansion Coefficient | \(in^3 \;/\; in^3\;F\) | \(mm^3 \;/\; mm^3\;C\) |
| \( V_o \) = Origional Volume | \(ft^3\) | \(m^3\) |
