Latent Heat
Latent Heat Formula |
||
|
\( Q_l \;=\; \dfrac{ Q }{ m }\) (Latent Heat) \( Q \;=\; Q_l \cdot m \) \( m \;=\; \dfrac{ Q }{ Q_l }\) |
||
| Symbol | English | Metric |
| \( Q_l \) = Latent Heat | \(Btu\;/\;lbm\) | \(kJ\;/\;kg\) |
| \( Q \) = Amount of Energy Transferred for the Phase Change | \(lbf-ft\) | \(J\) |
| \( m \) = Substance Mass | \(lbm\) | \(kg\) |
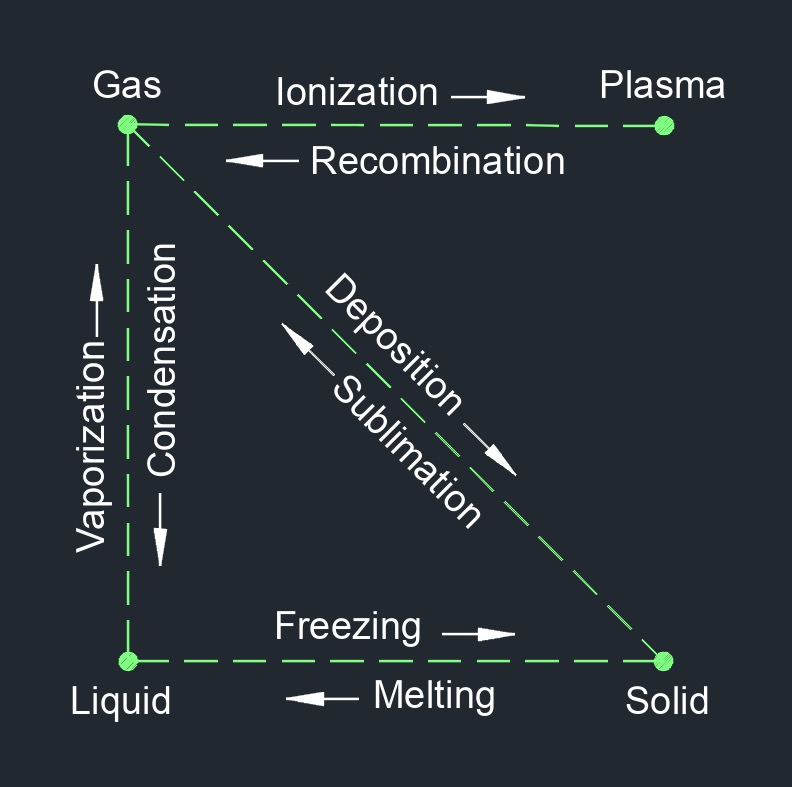
Latent heat, abbreviated as \(Q_l\), is the energy absorbed or released by a substance during a constant temperature or phase change from a solid to liquid, liquid to gas or vise versa. It is the heat energy associated with the transformation between different states of matter, such as solid to liquid (melting) or liquid to gas (vaporization). When a substance undergoes a phase change, the energy being added or removed is used to break or form intermolecular bonds rather than increasing or decreasing the temperature of the substance.
Latent heat plays a crucial role in various natural phenomena and everyday life. For example, during evaporation, water absorbs latent heat from the surroundings, causing cooling effects. This is why sweating cools our body as the water on our skin evaporates. Similarly, when water condenses into clouds, it releases latent heat, which can contribute to the formation of thunderstorms and other atmospheric processes.
Understanding the concept of latent heat is important in fields such as thermodynamics, meteorology, and climate science, as it influences the energy transfer and behavior of substances undergoing phase changes.

