Saturated Steam
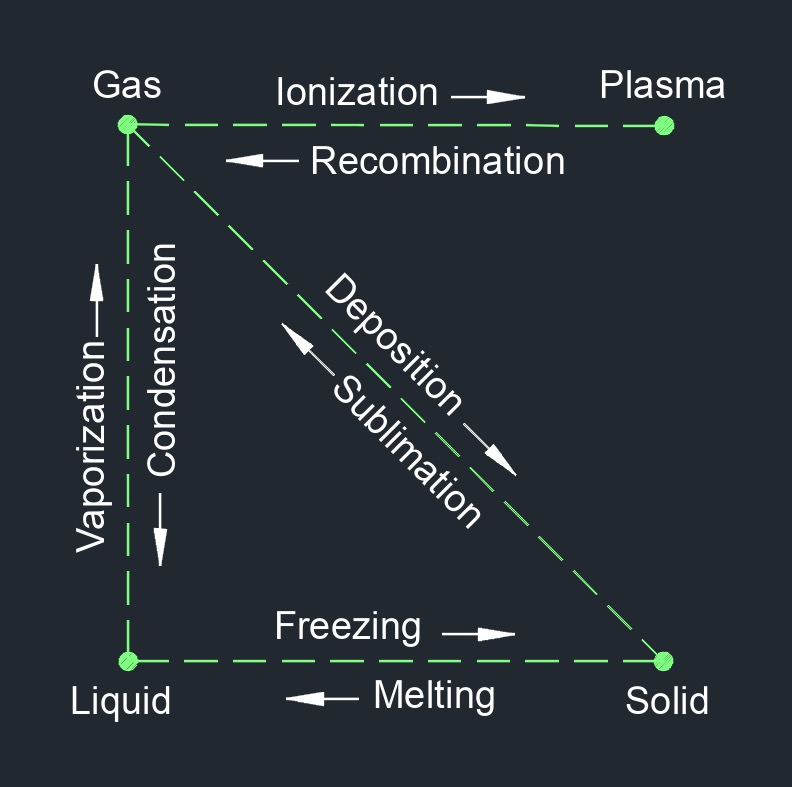 Saturated steam, no standard abbreviation, is steam that is in equilibrium with its own liquid state at a given temperature and pressure. It is the state of steam when it contains the maximum amount of moisture or water vapor for a given temperature and pressure. When water is heated, it undergoes a phase change from liquid to gas, becoming steam. As heat is applied, the temperature of the water rises until it reaches the boiling point at the given pressure. At this point, further heat input does not increase the temperature of the steam but converts more liquid water into vapor. The steam is said to be saturated because it is holding as much moisture as it can at that particular temperature and pressure.
Saturated steam, no standard abbreviation, is steam that is in equilibrium with its own liquid state at a given temperature and pressure. It is the state of steam when it contains the maximum amount of moisture or water vapor for a given temperature and pressure. When water is heated, it undergoes a phase change from liquid to gas, becoming steam. As heat is applied, the temperature of the water rises until it reaches the boiling point at the given pressure. At this point, further heat input does not increase the temperature of the steam but converts more liquid water into vapor. The steam is said to be saturated because it is holding as much moisture as it can at that particular temperature and pressure.
Saturated steam is often used in various industrial processes, such as power generation, heating systems, and chemical manufacturing. It carries a significant amount of heat energy and can be used for heating, driving turbines, or powering engines. It's worth noting that there is also superheated steam, which occurs when additional heat is added to saturated steam, raising its temperature above the boiling point. Superheated steam is typically utilized in power generation applications, where the high temperature steam is used to drive turbines for electricity production.

