Galvanic Anode (Sacrificial)
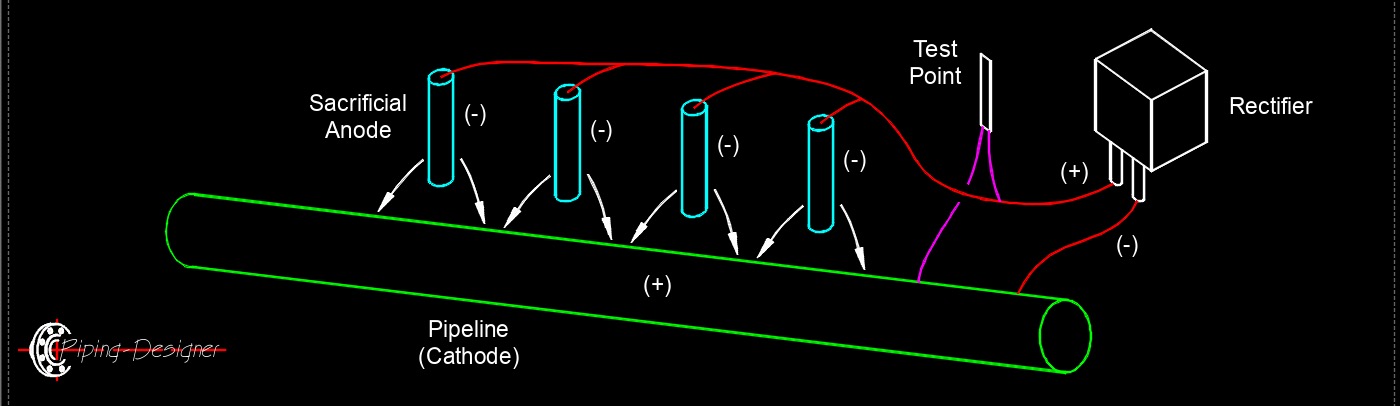 Galvanic (or sacrificial) anodes are a type of cathodic protection system used to prevent corrosion in metal structures, such as pipelines, ship hulls, and storage tanks. These anodes are made from metals that are more electrochemically active (i.e., more prone to corrosion) than the structure they protect, typically zinc, magnesium, or aluminum alloys. When connected to the structure in an electrolyte like soil or water, the anode corrodes preferentially, sacrificing itself by releasing electrons to the protected structure (the cathode). This electron flow prevents the structure from corroding by keeping it in a cathodic state. Sacrificial anodes are selected based on the environment, zinc for seawater, magnesium for soil or freshwater, and aluminum for marine applications due to its lightweight and high current output. They require no external power source, making them simple and cost-effective for smaller or less complex systems, but they must be replaced periodically as they corrode.
Galvanic (or sacrificial) anodes are a type of cathodic protection system used to prevent corrosion in metal structures, such as pipelines, ship hulls, and storage tanks. These anodes are made from metals that are more electrochemically active (i.e., more prone to corrosion) than the structure they protect, typically zinc, magnesium, or aluminum alloys. When connected to the structure in an electrolyte like soil or water, the anode corrodes preferentially, sacrificing itself by releasing electrons to the protected structure (the cathode). This electron flow prevents the structure from corroding by keeping it in a cathodic state. Sacrificial anodes are selected based on the environment, zinc for seawater, magnesium for soil or freshwater, and aluminum for marine applications due to its lightweight and high current output. They require no external power source, making them simple and cost-effective for smaller or less complex systems, but they must be replaced periodically as they corrode.Anode Materials
- Galvanic Anode (Sacrificial) - Galvanic anodes are made from metals that are more electrochemically active (less noble) than the metal they are protecting. When connected to the structure and immersed in an electrolyte (like soil or water), the galvanic anode corrodes preferentially, sacrificing itself to protect the structure. These systems don't require an external power source.
- Magnesium (Mg) - Magnesium has the most negative (active) potential among common galvanic anodes, making it suitable for environments with higher resistivity, such as onshore buried pipelines and certain freshwater applications. Primarily used for buried structures in soils and occasionally in freshwater tanks. Consumes relatively quickly, especially in low-resistivity environments, and can generate hydrogen gas.
- Zinc (Zn) - Zinc is less active than magnesium but more active than steel. It's effective in environments with lower resistivity, like seawater and brackish water, and low-resistivity soils. Widely used for marine pipelines, ship hulls, and in freshwater ballast tanks. Zinc ribbon anodes are also used for mitigating induced AC on buried pipelines. Performance can be negatively affected by temperatures above 60°C, where its polarity relative to steel can change.
- Aluminum (Al) - Aluminum alloys, often with small additions of zinc, indium, or mercury, are highly effective in seawater due to their good current output and high ampere-hour capacity. Commonly used for offshore structures, ship hulls, and marine pipelines. Requires specific alloying elements to prevent passivation (formation of a protective oxide layer that stops current flow) in some environments.

