Cathode
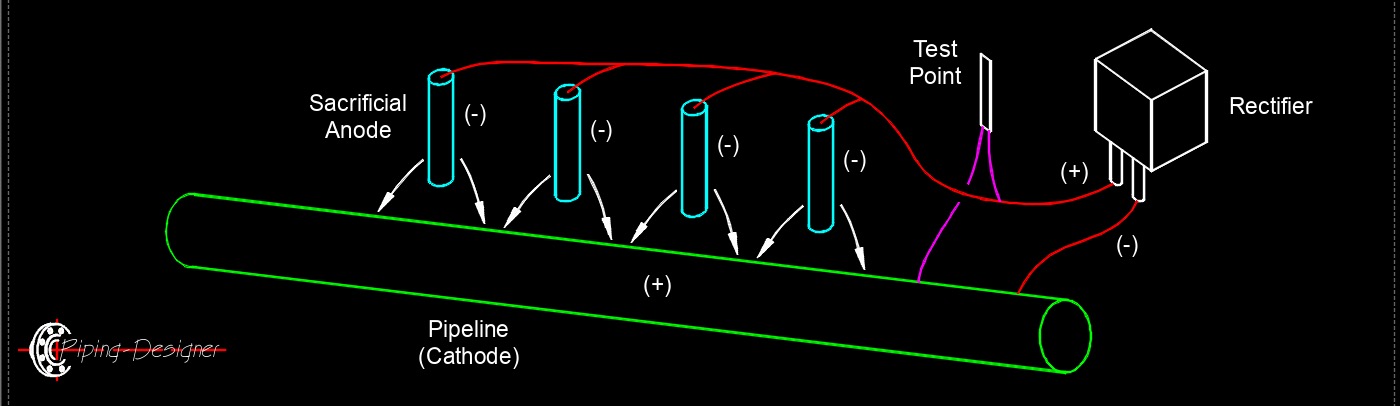 Cathode is the electrode in a cathodic protection system that receives electrons and is protected from corrosion. In this electrochemical process, the cathode is typically the metal structure, such as a pipeline, ship hull, or storage tank, that needs safeguarding against rust or other forms of corrosion. The system works by making the protected structure the cathode in an electrochemical cell, where it is connected to a more reactive sacrificial anode (like zinc or magnesium) or powered by an external DC current source in impressed current systems. Electrons flow to the cathode, reducing the metal's tendency to oxidize and corrode by counteracting the natural corrosion process. Essentially, the cathode is the component preserved from deterioration, as the anode corrodes instead or an external power source drives the protective current. This method is widely used in industries to extend the lifespan of metal infrastructure exposed to corrosive environments like soil or water.
Cathode is the electrode in a cathodic protection system that receives electrons and is protected from corrosion. In this electrochemical process, the cathode is typically the metal structure, such as a pipeline, ship hull, or storage tank, that needs safeguarding against rust or other forms of corrosion. The system works by making the protected structure the cathode in an electrochemical cell, where it is connected to a more reactive sacrificial anode (like zinc or magnesium) or powered by an external DC current source in impressed current systems. Electrons flow to the cathode, reducing the metal's tendency to oxidize and corrode by counteracting the natural corrosion process. Essentially, the cathode is the component preserved from deterioration, as the anode corrodes instead or an external power source drives the protective current. This method is widely used in industries to extend the lifespan of metal infrastructure exposed to corrosive environments like soil or water.

