Open, Closed, and Isolated Systems
Systems
In physics and engineering, a system is a defined collection of physical components or elements that are chosen to be analyzed together as a whole. A system can be as simple as a single object or as complex as an entire planet or galaxy.
In thermodynamics, a system is defined as a region of space or a quantity of matter that is chosen for study. The system can be either real or imaginary, and it can be separated from the rest of the universe by a boundary. The system boundary can either be stationary or moving. The boundary can be either real or imaginary, and it can be open, closed, or isolated, depending on the situation being studied.
The behavior of a system is characterized by its state variables, which are physical properties such as temperature, pressure, and volume. These state variables can change in response to various inputs or interactions, such as the addition or removal of heat or work, or the transfer of matter across the system boundary. Systems are studied in various fields of physics and engineering, including thermodynamics, mechanics, control theory, and electronics, among others. Understanding the behavior of systems is crucial for designing and analyzing complex physical processes, such as power generation, chemical reactions, and transportation systems.
| Transfer Permitted Types | |||
|---|---|---|---|
| Boundary Type | Transfer Type | ||
| Matter | Work | Heat | |
| Permeable to Matter | Yes | No | No |
| Permeable to Energy but
Impermeable to Matter |
No | Yes | Yes |
| Adiabatic | No | Yes | No |
| Adynamic and
Impermeable to Matter |
No | No | Yes |
| Isolating | No | No | No |
Boundary
The real or imaginary surface that separates the system ftom the surroundings and can be fixed or movable. In thermodynamics, a system boundary is an imaginary or real boundary that separates a thermodynamic system from its surroundings. The boundary defines what is included in the system and what is excluded, and it can be either open, closed, or isolated, depending on the type of system. The boundary can be imaginary, such as in the case of an ideal gas contained within a theoretical container with a fixed volume. In this case, the boundary is simply a mathematical construct that defines the limits of the system and separates it from its surroundings.
Alternatively, the boundary can be real, such as in the case of a sealed thermos containing hot coffee. In this case, the boundary is a physical barrier that separates the coffee and the air inside the thermos from the outside environment. The type of boundary chosen for a system depends on the specific situation being analyzed and the assumptions made about the system. For example, an open boundary may be chosen for a system if mass exchange with the surroundings is important, while a closed boundary may be used if only energy exchange is relevant. An isolated boundary may be used for theoretical calculations or idealized situations in which no exchange of matter or energy is allowed.
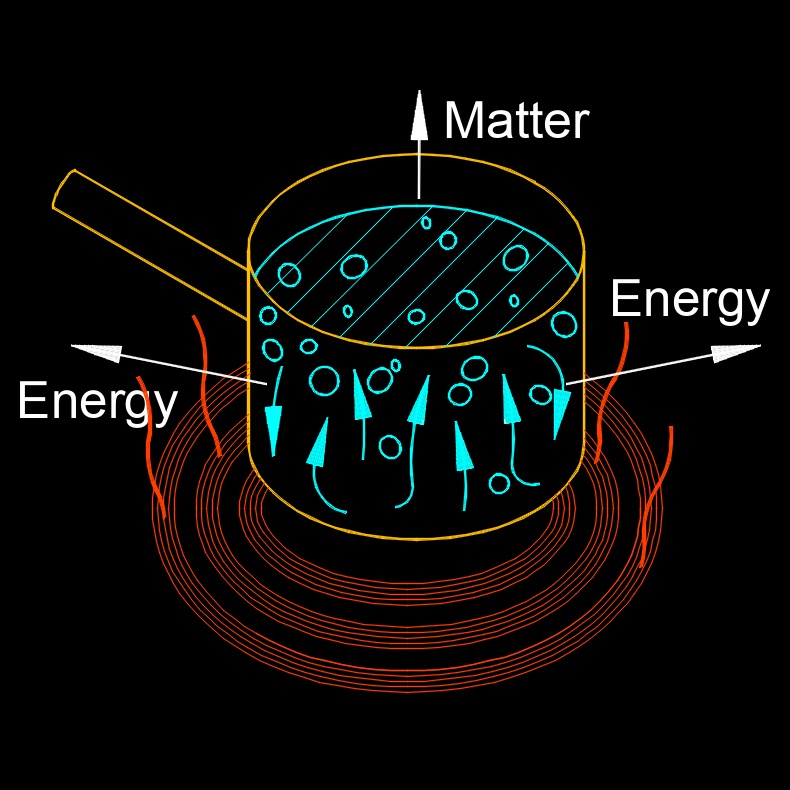 Open System
Open System
An open system is a system that can exchange both matter and energy with its surroundings. In other words, an open system is not isolated from its environment and can interact with it in various ways. This is in contrast to a closed system, which cannot exchange matter with its surroundings but can exchange energy. An open system can be found in many natural and human made systems, such as living organisms, ecosystems, industrial processes, and transportation systems. In an open system, matter can flow in and out of the system, and energy can be transferred into and out of the system as well.
For example, a plant is an open system because it takes in water, carbon dioxide, and nutrients from the environment, and releases oxygen and other waste products back into the environment. Similarly, a car engine is an open system because it takes in fuel and air from the environment, and releases exhaust gases and heat back into the environment.
Open systems are important in the study of thermodynamics and other areas of science and engineering because they often involve the transfer and transformation of energy and matter, which can have important effects on the behavior and performance of the system.
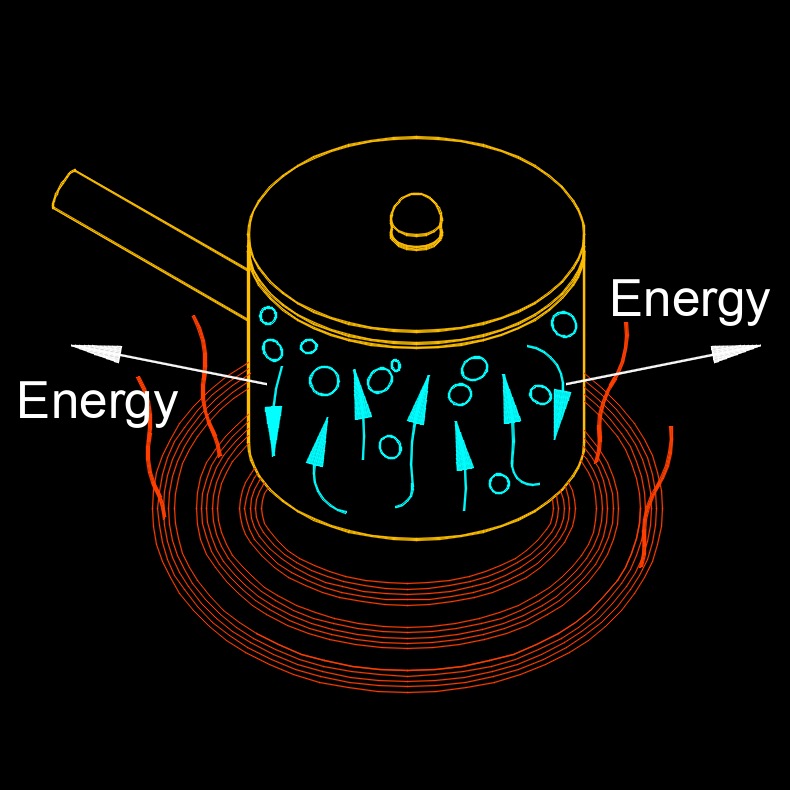 Closed System
Closed System
A closed system is a system that can exchange energy with its surroundings, but cannot exchange matter. In other words, a closed system is isolated from its environment in terms of matter exchange, but can still exchange energy with it. A closed system can be found in many natural and human-made systems, such as a sealed container with a fixed amount of gas, the Earth's atmosphere, or a piston-cylinder system used in thermodynamics. In a closed system, the total amount of matter within the system remains constant, but energy can be transferred into or out of the system in various forms, such as heat, work, or radiation.
For example, a sealed container filled with a fixed amount of gas is a closed system. The gas molecules can interact with each other and with the walls of the container, but no matter can enter or leave the system. The temperature and pressure of the gas can change due to energy transfer, but the total mass of the gas remains constant.
Closed systems are important in the study of thermodynamics because they allow for the analysis of energy transfer and transformation within a system, without the complications of matter exchange with the environment. They are also useful in engineering and other fields where it is necessary to control or maintain the properties of a system in a closed environment.
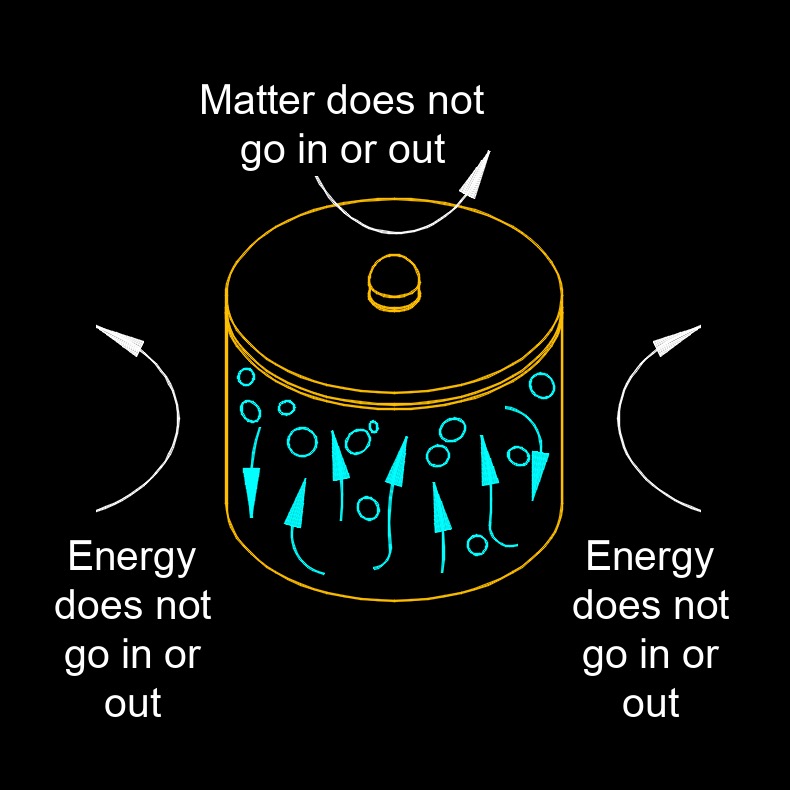 Isolated System
Isolated System
An isolated system is a thermodynamic system that does not exchange matter or energy with its surroundings. In other words, an isolated system is completely closed off from its environment and cannot transfer either matter or energy across its boundary. In practice, true isolated systems are very rare, as almost all physical systems interact with their environment to some degree. However, for the purposes of theoretical analysis, it is often useful to consider systems that are isolated in order to simplify the analysis of their behavior.
One example of an isolated system is the universe as a whole. The universe is often considered to be a closed, self contained system that cannot exchange matter or energy with anything outside of it. While this may be an idealization, it provides a useful framework for understanding the behavior of the universe on a large scale. Another example of an isolated system is a perfectly insulated thermos containing hot coffee. In this case, the thermos is designed to minimize the transfer of heat energy between the coffee and the environment, making it effectively an isolated system for the purpose of heat transfer.
Isolated systems are important in thermodynamics and other areas of physics because they provide a simplified framework for analyzing the behavior of physical systems under idealized conditions.

