Density of an Element
Density of an Element |
||
| Element Name | Element Symbol | Density \( g \;/\; cm^3 \) |
| Actinium | Ac | 10.07 |
| Aluminum | Al | 2.7 |
| Americium | Am | 13.67 |
| Antimony | Sb | 6.697 |
| Argon | Ar | 1.784 |
| Arsenic | As | 5.727 |
| Astatine | At | N/A |
| Barium | Ba | 3.51 |
| Berkelium | Bk | 14.78 |
| Beryllium | Be | 41.848 |
| Bismuth | Bi | 9.78 |
| Bohrium | Bh | N/A |
| Boron | B | 2.46 |
| Bromine | Br | 3.12 |
| Cadmium | Cd | 8.65 |
| Caesium | Cs | N/A |
| Calcium | Ca | 1.55 |
| Californium | Cf | 15.1 |
| Carbon | C | 2.26 |
| Cerium | Ce | 6.689 |
| Chlorine | Cl | 3.214 |
| Chromium | Cr | 7.91 |
| Cobalt | Co | 8.9 |
| Copper | Cu | 8.96 |
| Curium | Cm | 13.51 |
| Darmstadtium | Ds | N/A |
| Dubnium | Db | N/A |
| Dysprosium | Dy | 8.551 |
| Einsteinium | Es | N/A |
| Erbium | Er | 9.066 |
| Europium | Eu | 5.244 |
| Fermium | Fm | N/A |
| Fluorine | F | N/A |
| Francium | Fr | N/A |
| Gadolinium | Gd | 7.901 |
| Gallium | Ga | 5.904 |
| Germanium | Ge | 5.323 |
| Gold | Au | 19.3 |
| Hahnium | Hf | 13.31 |
| Hassium | Hs | N/A |
| Helium | He | 0.1785 |
| Holmium | Ho | 8.795 |
| Hydrogen | H | 0.0899 |
| Indium | In | 7.31 |
| Iodine | I | 4.94 |
| Iridium | Ir | 22.56 |
| Iron | Fe | 7.874 |
| Krypton | Kr | 3.75 |
| Lanthanum | La | 6.146 |
| Lawrencium | Lr | N/A |
| Lead | Pb | 11.34 |
| Lithium | Li | 0.535 |
| Lutetium | Lu | 9.841 |
| Magnesium | Mg | 1.738 |
| Manganese | Mn | 7.47 |
| Meitnerium | Mt | N/A |
| Mendelevium | Md | N/A |
| Mercury | Hg | 13.534 |
| Molybdenum | Mo | 10.28 |
| Neodymium | Nd | 7.01 |
| Neon | Ne | 0.9 |
| Neptunium | Np | 20.45 |
| Nickel | Ni | 8.908 |
| Niobium | Nb | 8.57 |
| Nitrogen | N | 1.251 |
| Nobelium | No | N/A |
| Osmium | Os | 22.59 |
| Oxygen | O | 1.429 |
| Palladium | Pd | 12.023 |
| Phosphorus | P | 1.823 |
| Platinum | Pt | 12.45 |
| Plutonium | Pu | 19.816 |
| Polonium | Po | 9.126 |
| Potassium | K | 0.856 |
| Praseodymium | Pr | 6.64 |
| Promethium | Pm | 7.264 |
| Protactinium | Pa | 15.37 |
| Radium | Ra | 5 |
| Radon | Rn | 9.73 |
| Rhenium | Re | 21.02 |
| Rhodium | Rh | 12.45 |
| Roentgenium | Rg | N/A |
| Rubidium | Rb | 1.532 |
| Ruthenium | Ru | 12.37 |
| Rutherfordium | Rf | N/A |
| Samarium | Sm | 7.353 |
| Scandium | Sc | 2.985 |
| Seaborgium | Sg | N/A |
| Selenium | Se | 4.819 |
| Silicon | Si | 2.33 |
| Silver | Ag | 10.49 |
| Sodium | Na | 0.968 |
| Strontium | Sr | 2.63 |
| Sulfur | S | 1.96 |
| Tantalum | Ta | 16.65 |
| Technetium | Tc | 11.5 |
| Tellurium | Te | 6.24 |
| Terbium | Tb | 8.219 |
| Thallium | Tl | 11.85 |
| Thorium | Th | 11.724 |
| Thulium | Tm | 9.32 |
| Tin | Sn | 7.31 |
| Titanium | Ti | 4.507 |
| Tungsten | W | 19.25 |
| Ununbium | Uub | N/A |
| Ununhexium | Uuh | N/A |
| Ununoctium | Uuo | N/A |
| Ununpentium | Uup | N/A |
| Ununquadium | Uuq | N/A |
| Ununseptium | Uus | N/A |
| Ununtrium | Uut | N/A |
| Uranium | U | 19.05 |
| Vanadium | V | 6.11 |
| Xenon | Xe | 5.9 |
| Ytterbium | Yb | 6.57 |
| Yttrium | Y | 4.472 |
| Zinc | Zn | 7.14 |
| Zirconium | Zr | 6.511 |
| Element Name | Element Symbol | Density \(g\;/\;cm^3 \) |
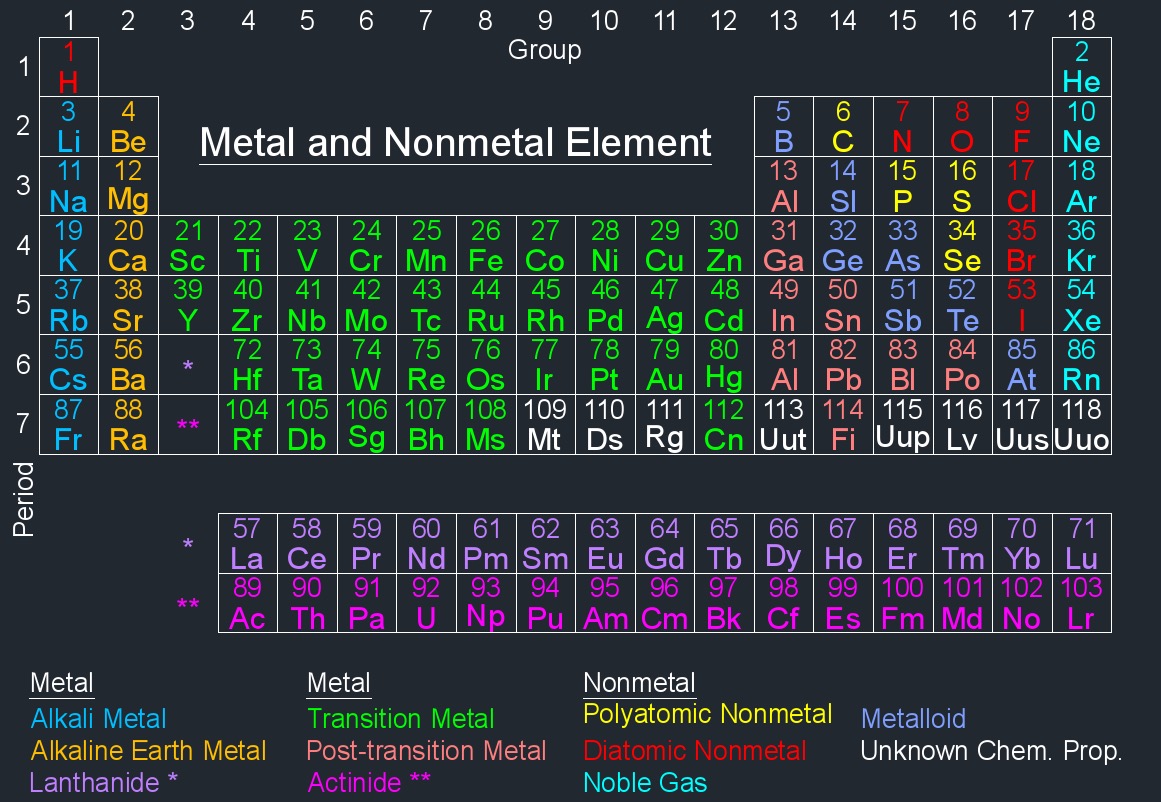
Density of an element is a physical property that relates the mass of a given amount of the element to its volume. Density can vary widely among different elements due to differences in atomic mass and atomic packing. In general, metals tend to have higher densities than nonmetals because of the close packing of atoms in metallic structures. It's important to note that the density of an element can be influenced by external factors such as temperature and pressure. Therefore, when specifying the density of a material, it's common to include the conditions under which the measurement was taken (density at room temperature and atmospheric pressure).
The density of an element is a useful parameter in various scientific and engineering applications. It can provide insights into the elemental composition of materials and is often used in calculations related to buoyancy, fluid dynamics, and material characterization.