Saturated Vapor Pressure
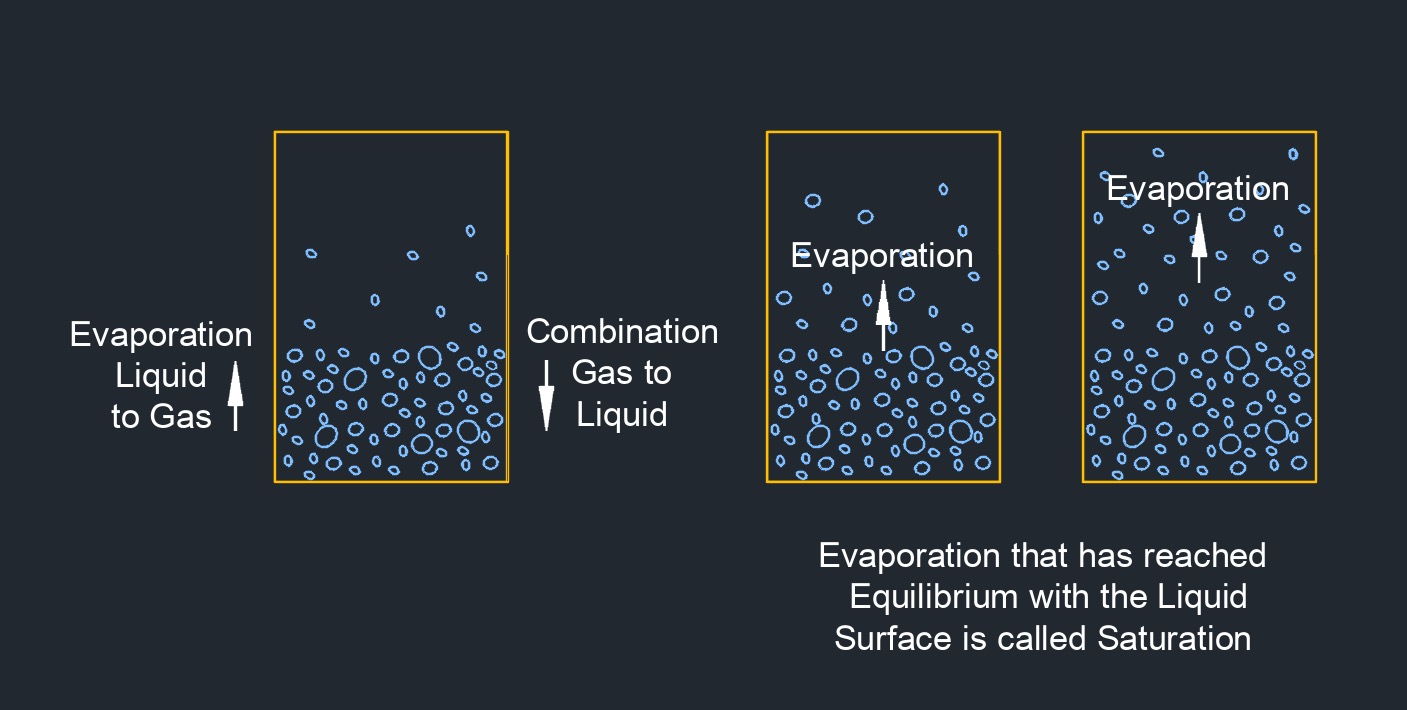 Saturated vapor pressure, abbreviated as \(e_s\), is the pressure exerted by the vapor molecules of a substance in equilibrium with its liquid or solid phase at a specific temperature. It represents the maximum vapor pressure that a substance can achieve at that temperature, meaning that further increase in vapor pressure would lead to condensation or saturation of the vapor. The saturated vapor pressure is directly related to the temperature and the properties of the substance. As the temperature increases, the saturated vapor pressure generally increases because more molecules gain sufficient energy to transition from the liquid or solid phase to the vapor phase.
Saturated vapor pressure, abbreviated as \(e_s\), is the pressure exerted by the vapor molecules of a substance in equilibrium with its liquid or solid phase at a specific temperature. It represents the maximum vapor pressure that a substance can achieve at that temperature, meaning that further increase in vapor pressure would lead to condensation or saturation of the vapor. The saturated vapor pressure is directly related to the temperature and the properties of the substance. As the temperature increases, the saturated vapor pressure generally increases because more molecules gain sufficient energy to transition from the liquid or solid phase to the vapor phase.
The saturated vapor pressure is a crucial parameter in various applications, including meteorology, chemistry, and engineering. In meteorology, it is used to determine the moisture content and potential for condensation or cloud formation in the atmosphere. In chemistry and engineering, the saturated vapor pressure is utilized in calculations involving vapor-liquid equilibrium, such as distillation, evaporation, and drying processes.
The saturated vapor pressure of a substance can be measured experimentally using various methods, including the use of vapor pressure thermometers or devices specifically designed for vapor pressure determination. It can also be estimated or predicted using mathematical models and correlations based on the properties of the substance, such as Antoine's equation or the Clausius-Clapeyron equation.

