Chemical Engineering
Materials, Chemical, Engineering, Chemical Elements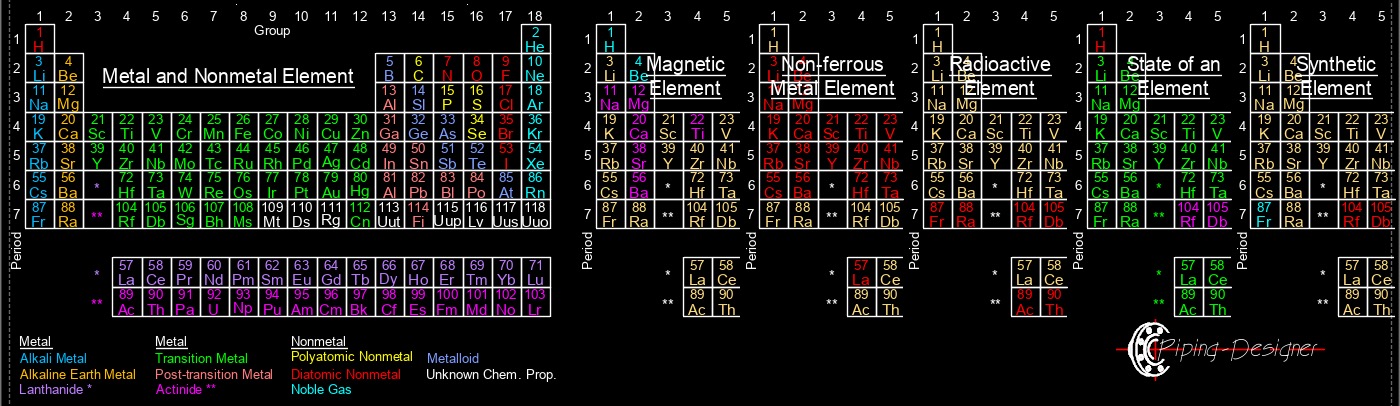
Chemical engineering and chemistry are really two different disciplines. Chemical engineering is the general knowledge of chemistry but with a focus on mathmatics, fluid dynamics, and thermodynamics. Chemistry is a fundamental knowledge in the chemical properties, reactions, and their principles and theories. Chemical engineers work in a variety of industries, including pharmaceuticals, food and beverage, energy, and materials processing. They may work in research and development, process design and optimization, production, or process control, depending on their area of specialization.
| Engineering |
| Chemical Engineering |
You probably think that chemistry is part of physics, but it is not. Both are part of physical science, but different branches, and both study the structure and properties of matter. Physics deals with understand the universe in a fundamental way. Chemistry is how substances interact with each other.
- See Article - Chemical Glossary
Chemical Engineering Type
Biomechanical Engineering - Ths is a profession combining the principles of mechanical engineering and biology to design and develop medical devices, prosthetic limbs, and other equipment used in the medical field. They use their knowledge of mechanics, materials science, and biology to understand how the human body moves and how it is affected by disease or injury. They work to improve the function of medical devices and equipment, and to develop new technologies that can enhance the quality of life for people with disabilities or chronic illnesses.
Biomolecular Engineering - The field of biomolecular engineering takes the study of biochemistry and molecular biology and translates the knowledge into creating processes, devices and products that benefit people. Biomolecular engineers have been responsible for developing live saving treatments and drugs. Some biomolecular engineers work to create alternative fuel sources that provide the energy we need while still protecting the environment. By creating new molecules and harnessing the power of current ones, biomolecular engineers can take new ideas from the beaker to mass market.
Bioprocess Engineering - Bioprocess engineers design and optimize bioprocesses for the production of biopharmaceuticals, such as vaccines, antibodies and gene therapies. They work on the development of new bioprocesses and ensure that the production process is compliant with regulatory guidelines. They also work on the production and optimization of systems like bioreactors and purification systems. Bioprocess engineers may have some additional collaboration with other molecular biologists, biochemists and pharmacists, to develop innovative biopharmaceutical products.
Food Processing Engineering - They create food processing systems, ensuring that they're safe, efficient and of high quality. They evaluate existing processes, identify areas for improvement and develop new processes to increase productivity and reduce costs. Additionally, they monitor and troubleshoot food production processes, ensuring that they work to solve any issues that arise. Some food process engineers may also aid in the development of new food products. This is when the main chemical engineering duties may arise in this job.
Material Science Engineering - They work to understand the fundamental physical origins of material behavior in order to optimize properties of existing materials through structure modification and processing, design and invent new and better materials, and understand why some materials unexpectedly fail. Materials engineers create and manufacture new materials, which include semiconductors, polymers, ceramics, ferrous and non-ferrous alloys, and composite materials. Their contributions have led to breakthroughs in microelectronics, displays, energy storage, aerospace, and biomedical devices, among many other fields.
Petrochemical Engineering - These engineers research and develop new ways to decompose oil and petroleum to create new ways to produce petrochemicals. Their duties include the management of a plant or a refinery’s production process and applying research and development in chemistry labs. The field of petrochemical engineering is mainly solution driven and problem solving.
Pharmaceutical Engineering - They create and utilize pharmaceutical manufacturing processes, ensuring that they produce the drugs safely, efficiently and at a high level of quality. They work on the development of new drugs and ensure that the manufacturing process is compliant with regulatory guidelines. Additionally, they work on the production and optimization of drug delivery systems and packaging. Pharmaceutical engineers also collaborate with other professionals, such as chemists, biologists and pharmacologists, to develop innovative drug products.

