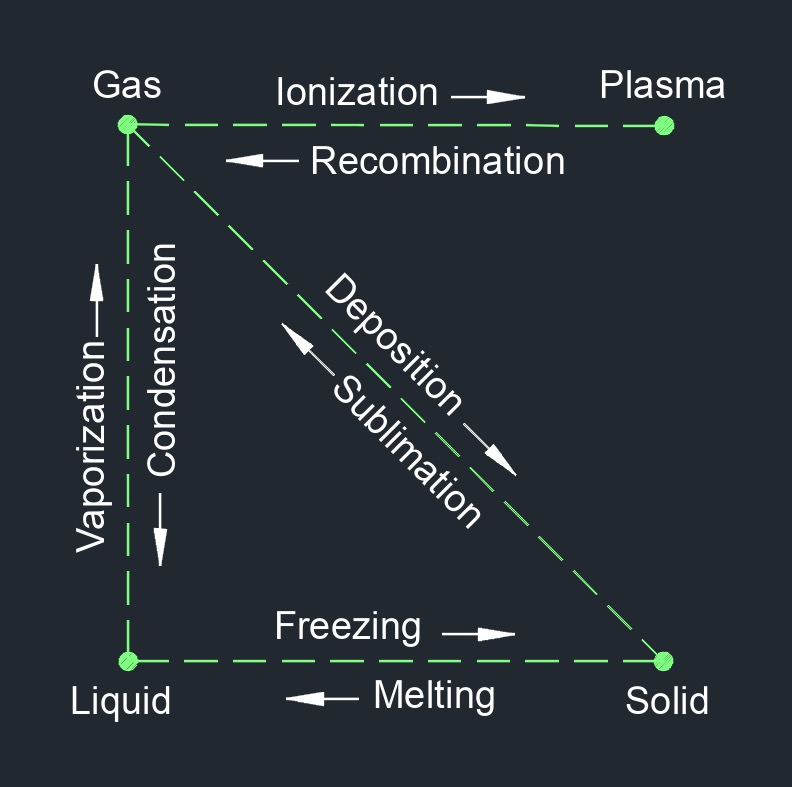
 Steam, abbreviated as \(STM\), is the invisible vapor (gas) when water is heated to its boiling point and passes from a liquid to a gaseous state. As water is heated and approaches its boiling point, some of the molecules attain kinetic energy enough to escape into the space above the surface of the liquid. The more the water is heated the more molecules excapes. When more molecules leave the liquid than enter the liquid, the saturation point is reached. As the temperature continues to rising it reaches superheated steam where no liquid exists. The temperature at which water boils and turns into steam depends on the pressure applied to it. At standard atmospheric pressure (1 atmosphere or 101.3 kilopascals), water boils at 100 degrees Celsius (212 degrees Fahrenheit), resulting in the formation of steam.
Steam, abbreviated as \(STM\), is the invisible vapor (gas) when water is heated to its boiling point and passes from a liquid to a gaseous state. As water is heated and approaches its boiling point, some of the molecules attain kinetic energy enough to escape into the space above the surface of the liquid. The more the water is heated the more molecules excapes. When more molecules leave the liquid than enter the liquid, the saturation point is reached. As the temperature continues to rising it reaches superheated steam where no liquid exists. The temperature at which water boils and turns into steam depends on the pressure applied to it. At standard atmospheric pressure (1 atmosphere or 101.3 kilopascals), water boils at 100 degrees Celsius (212 degrees Fahrenheit), resulting in the formation of steam.
Steam has several unique properties and behaviors. It occupies a greater volume than an equivalent mass of liquid water, as the gas molecules are more spread out and less densely packed. Steam is also less dense than air, making it rise and disperse quickly.
In addition to its applications in industrial processes and power generation, steam has played a crucial role in the development of thermodynamics and the study of heat transfer. Steam engines, for instance, were historically significant in powering trains and other machinery during the industrial revolution. Understanding the physics of steam involves concepts such as phase transitions, thermodynamics, heat transfer, and the behavior of gases. It has practical implications in fields like engineering, energy production, and environmental science.
Wet Steam (Unsaturated Steam) \(\;\;100 \; ^\circ C\) \((212 \; ^\circ F)\)
Ice \(\;\;0 \; ^\circ C\) \((32 \; ^\circ F)\)
Steam Superheated Vapor Properties Table
Steam Types
These steam types have different characteristics and are used in various industrial, commercial, and residential applications depending on the specific requirements of the process or system.
Dry Steam - Dry steam is steam that contains no liquid water and is completely vaporized. It is typically preferred for many applications because it provides more efficient heat transfer and avoids issues associated with wet steam.
Saturated Steam - Saturated steam is steam that is in equilibrium with liquid water at a given
pressure and
temperature. It contains the maximum amount of moisture possible at that pressure and temperature. Saturated steam is commonly used for heating, power generation, and industrial processes.
Wet Steam - Wet steam, also called saturated steam with liquid droplets, is a mixture of vapor and liquid water. It occurs when saturated steam is partially condensed due to cooling or expansion. Wet steam can be problematic in some applications as the liquid droplets can cause erosion and damage to equipment.
Steam Quality
Steam quality, also called steam purity or dryness fraction, is the amount of moisture or
liquid water present in
steam. It is a measure of how "dry" or "wet" the steam is.
Steam quality is expressed as a percentage or a fraction and represents the ratio of the
mass of steam to the total mass of steam and water (steam + water) in a given sample. A steam quality of 100% indicates completely dry or saturated steam with no liquid water present, while a steam quality of 0% indicates that the steam is entirely composed of liquid water.
Steam quality is an important parameter in various industrial processes and applications. In some cases, such as power generation or steam turbine operation, it is crucial to have dry or high quality steam to ensure efficient and reliable performance. Wet or low quality steam with excessive moisture can cause issues like erosion,
corrosion, and reduced
heat transfer efficiency.
The measurement and control of steam quality are typically performed using specialized instruments called steam quality meters or separators. These devices separate the liquid and vapor phases of steam and determine the percentage of moisture content present. Various factors can affect steam quality, including the design and operation of steam generation systems, boiler performance, water treatment, and steam distribution. Proper maintenance and control of these factors are essential to achieve and maintain the desired steam quality for specific applications.
Steam Uses
Steam is used for many things in piping design. In the oil and gas industry, steam is used to heat production tanks, keep flow lines from freezing and to increase oil production in formations that are very viscious. In other industries, it can be used to sterilize & clean equipment, sterilize equipment, and in reboilers which maintain tight tolerances on their temperatures. Because of this, it is important to ensure that the line has been
sized properly to reduce
pressure drop and to also ensure that right
steam quality,
pressure and
temperature is being delivered where it is supposed to. Sizing a line too small will cause the water vapor and liquid to travel too fast which will reduce overall pressure in the line. If the line is too large, the fluid and vapor will travel slower and heat loss will occur. Equally important to sizing a line,
sizing a valve for use in steam service.
Steam has a wide range of applications across various industries due to its unique properties, including its ability to carry large amounts of
heat energy and its versatility.

 Steam, abbreviated as \(STM\), is the invisible vapor (gas) when water is heated to its boiling point and passes from a liquid to a gaseous state. As water is heated and approaches its boiling point, some of the molecules attain kinetic energy enough to escape into the space above the surface of the liquid. The more the water is heated the more molecules excapes. When more molecules leave the liquid than enter the liquid, the saturation point is reached. As the temperature continues to rising it reaches superheated steam where no liquid exists. The temperature at which water boils and turns into steam depends on the pressure applied to it. At standard atmospheric pressure (1 atmosphere or 101.3 kilopascals), water boils at 100 degrees Celsius (212 degrees Fahrenheit), resulting in the formation of steam.
Steam, abbreviated as \(STM\), is the invisible vapor (gas) when water is heated to its boiling point and passes from a liquid to a gaseous state. As water is heated and approaches its boiling point, some of the molecules attain kinetic energy enough to escape into the space above the surface of the liquid. The more the water is heated the more molecules excapes. When more molecules leave the liquid than enter the liquid, the saturation point is reached. As the temperature continues to rising it reaches superheated steam where no liquid exists. The temperature at which water boils and turns into steam depends on the pressure applied to it. At standard atmospheric pressure (1 atmosphere or 101.3 kilopascals), water boils at 100 degrees Celsius (212 degrees Fahrenheit), resulting in the formation of steam.
