Entropy
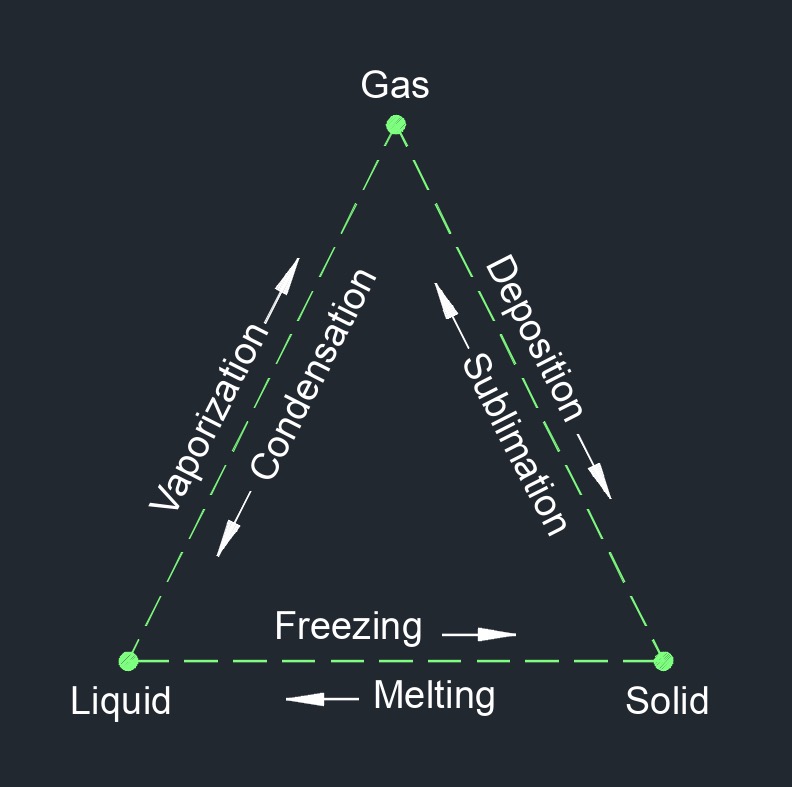 Entropy, abbreviated as \(S\), (English unit \(\frac{Btu}{lbm-R}\), Metric unit \(\frac{kJ}{kg-K}\)), measures the unavailable energy in a heat system. It is a measure of the disorder or randomness of a system and of its constituent molecules. In thermodynamics it is a measure of the unavailability of a system's energy fo do work. The concept of entropy is closely related to the Second Law of Thermodynamics, which states that the total entropy of a closed system can never decrease over time. This means that, in a closed system, any energy that is converted from one form to another will increase the overall entropy of the system.
Entropy, abbreviated as \(S\), (English unit \(\frac{Btu}{lbm-R}\), Metric unit \(\frac{kJ}{kg-K}\)), measures the unavailable energy in a heat system. It is a measure of the disorder or randomness of a system and of its constituent molecules. In thermodynamics it is a measure of the unavailability of a system's energy fo do work. The concept of entropy is closely related to the Second Law of Thermodynamics, which states that the total entropy of a closed system can never decrease over time. This means that, in a closed system, any energy that is converted from one form to another will increase the overall entropy of the system.
Entropy has many applications in science and engineering, including in the study of chemical reactions, the behavior of materials, and the design of thermodynamic systems such as engines and refrigerators. It is a fundamental concept in physics and is used to describe the behavior of complex systems across many fields of science and engineering.

