Coulomb's Law
Coulomb's Law Formula |
||
|
\( F_e \;=\; k_e \cdot \dfrac{ q_1 \cdot q_2 }{ r^2 } \) (Coulomb's Law) \( k_e \;=\; \dfrac{ F_e \cdot r^2 }{ q_1 \cdot q_2 }\) \( q_1 \;=\; \dfrac{ F_e \cdot r^2 }{ k_e \cdot q_2 }\) \( q_2 \;=\; \dfrac{ F_e \cdot r^2 }{ k_e \cdot q_1 }\) \( r \;=\; \sqrt{ \dfrac{ k_e \cdot q_1 \cdot q_2 }{ F_e } }\) |
||
| Symbol | English | Metric |
| \( F_e \) = Electrostatic Force | \(lbf\) | \(N\) |
| \( k_e \) = Coulomb's Constant (see Physics Constants) | \(lbf-ft^2 \;/\; F^2\) | \(N-m^2 \;/\; C^2\) |
| \( q_1 \) = Electric Charge | \(C\) | \(C\) |
| \( q_2 \) = Electric Charge | \(C\) | \(C\) |
| \( r \) = Distance Separating the Centers of the Two Charges | \(ft\) | \(m\) |
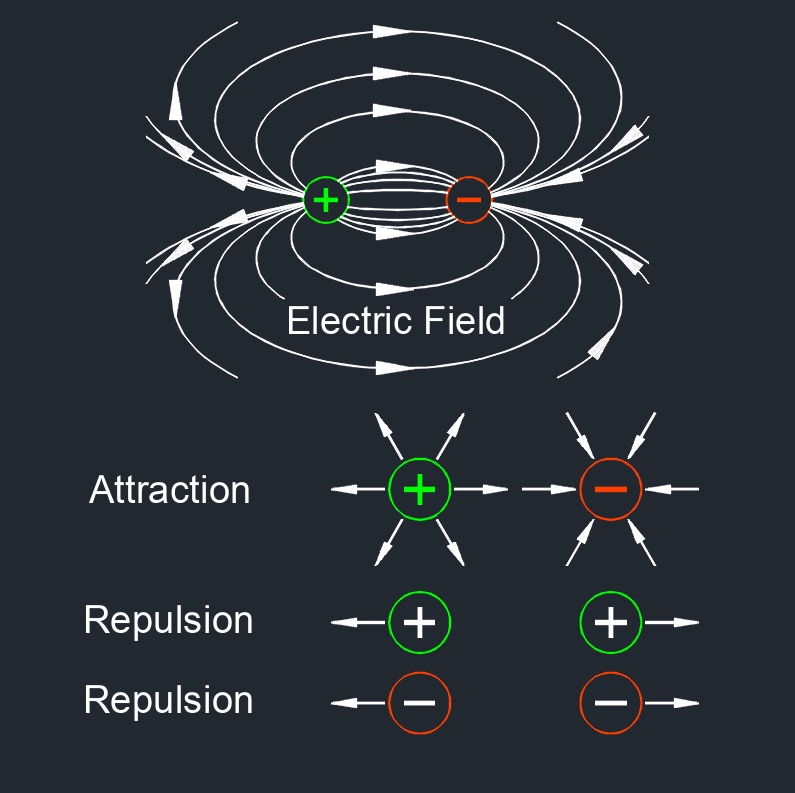
Coulomb's law is the magnitude of the electrostatic force between two electric charges. In case of more than two electric charges, the superposition principle in addition to coulomb’s law is applied to calculate the net electronic force acting on any one charge.
 According to this superposition principle, the total force acting on a given charge is equal to the vector sum of forces exerted on it by all the other charges. The force between two charges can be attractive if the charges have opposite signs (one positive and one negative), or repulsive if the charges have the same sign (both positive or both negative). The force follows an inverse square law, meaning that doubling the distance between charges reduces the force by a factor of four, while halving the distance increases the force by a factor of four.
According to this superposition principle, the total force acting on a given charge is equal to the vector sum of forces exerted on it by all the other charges. The force between two charges can be attractive if the charges have opposite signs (one positive and one negative), or repulsive if the charges have the same sign (both positive or both negative). The force follows an inverse square law, meaning that doubling the distance between charges reduces the force by a factor of four, while halving the distance increases the force by a factor of four.
Coulomb's law is fundamental to the understanding of electric forces and plays a crucial role in various fields, including electromagnetism, electrical engineering, and physics. It provides a mathematica description of the force between charged particles and enables the calculation of electric forces and interactions in different systems.

